- Record: found
- Abstract: found
- Article: found
Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration
Read this article at
Abstract
Background
Peripheral cold neuropathic pain is a serious side effect of oxaliplatin treatment. However, the mechanism of oxaliplatin-induced cold hyperalgesia is unknown. In the present study, we investigated the effects of oxaliplatin on transient receptor potential ankyrin 1 (TRPA1) in dorsal root ganglion (DRG) neurons of rats.
Results
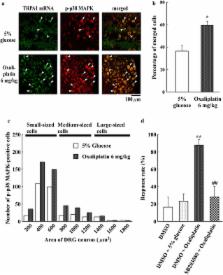
Behavioral assessment using the acetone spray test showed that 3 and 6 mg/kg oxaliplatin (i.p.) induced acute cold hypersensitivity after 1, 2, 4, and 7 days. Real-time PCR showed that oxaliplatin (6 mg/kg) significantly increased TRPA1 mRNA expression in DRGs at days 1, 2, and 4. Western blotting revealed that oxaliplatin significantly increased TRPA1 protein expression in DRGs at days 2, 4, and 7. Moreover, in situ hybridization histochemistry revealed that most TRPA1 mRNA-labeled neurons in the DRGs were small in size. Oxaliplatin significantly increased co-localization of TRPA1 expression and isolectin B4 binding in DRG neurons. Oxaliplatin induced a significant increase in the percent of TRPA1 mRNA-positive small neurons in DRGs at days 1, 2, and 4. In addition, we found that intrathecal administration of TRPA1 antisense, but not TRPA1 mismatched oligodeoxynucleotides, knocked down TRPA1 expression and decreased oxaliplatin-induced cold hyperalgesia. Double labeling showed that p-p38 mitogen-activated protein kinase (MAPK) was co-expressed in TRPA1 mRNA-labeled neurons at day 2 after oxaliplatin administration. Intrathecal administration of the p38 MAPK inhibitor, SB203580, significantly decreased oxaliplatin-induced acute cold hypersensitivity.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
A TRP channel that senses cold stimuli and menthol.
- Record: found
- Abstract: found
- Article: not found
Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin.
- Record: found
- Abstract: found
- Article: not found