- Record: found
- Abstract: found
- Article: found
Efficacy and safety of rituximab in the treatment of membranous nephropathy : A systematic review and meta-analysis
Read this article at
Abstract
Background and objectives:
Rituximab (RTX) is considered to be a promising drug for curing membranous nephropathy. However, the efficacy and safety of RTX in treating membranous nephropathy remain uncertain. This meta-analysis aimed to investigate the efficacy and safety of RTX in patients with membranous nephropathy.
Methods:
A literature search was performed using Pubmed, Embase, OVID, and Cochrane Library and randomized controlled trials (RCTs) case-controls and cohort studies published till 30 July 2019 were assessed. The studies assessing the efficacy and safety of RTX in patients with membranous nephropathy were included.
Results:
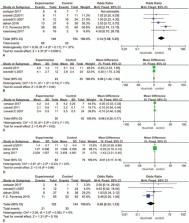
Eight relevant trials involving 542 patients were included in the meta-analysis. It was found that RTX did not significantly improve serum albumin levels and e-GFR when compared with the control group (including cyclosporine and cyclophosphamide, chlorambucil, prednisone, non-immunosuppressive anti-proteinuria treatment), serum albumin levels (OR = 0.31, 95%CI–0.12–0.74, P = .15), e-GFR (OR = –1.49, 95%CI–17.14–14.17, P = .85). However, RTX did reduce the serum creatinine (OR = –0.01, 95%CI–0.36–0.34, P = .95) and urinary protein (OR = –2.39, 95%CI –7.30 –2.53, P = .34) levels. Also, in comparison to the control group, RTX did improve the total remission rate (OR = 1.63, 95%CI 0.48–5.54, P = .43), achieve a higher rate of complete remission (OR = 2.54, 95%CI 1.65–3.90, P < .01) and also reduced the amount of M-type phospholipase A2 receptor-Antibody depletion in patients (OR = 5.59, 95%CI 1.81–17.2, P = .003). RTX-related adverse events were mostly mild (most infusion-related reactions) in nature and serious adverse events were rare.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Primary Membranous Nephropathy.
- Record: found
- Abstract: found
- Article: not found
Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up.
- Record: found
- Abstract: found
- Article: not found