Introduction
In November 2013 the American College of Cardiology (ACC) and the American Heart Association (AHA) issued four new guidelines for atherosclerotic cardiovascular disease (ASCVD) prevention that focused on cardiovascular risk assessment [1], lifestyle management [2], obesity management [3], and blood cholesterol management [4]. These guidelines were unique in that they were based nearly exclusively on higher-quality randomized controlled clinical trials or systematic reviews and meta-analyses, with less “expert opinion” than used in prior guidelines. Each was designed to answer specific critical questions rather than to broadly address the topic at hand. This article provides an overview of the ACC/AHA guideline for blood cholesterol management [4], including recent updates that consider the use of nonstatin therapies, as well as the foundational role of the ASCVD risk assessment, lifestyle management, and obesity management guidelines. These guidelines provide the practitioner with an overall approach to preventive cardiology. The potential role of cholesterol absorption inhibition and, in particular, proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody (mAb) therapy in addressing residual ASCVD risk is also discussed.
Cardiovascular Risk Assessment, Lifestyle Management, and Obesity Management Guidelines
Global risk assessment by risk scores or risk functions to evaluate a person’s ASCVD risk is the basis of preventive cardiology and the key to appropriate targeting of lipid and other preventive therapies for primary prevention of ASCVD. As early as 1976, William B. Kannel of the Framingham Heart Study noted that risk functions provide an “economic and efficient method for identifying persons at high cardiovascular risk who need preventive treatment” [5], but it was not until 20 years later that the ACC Bethesda Conference noted that the intensity of treatment should match a person’s risk [6]. Risk scores are useful to communicate future hazard of ASCVD in patients and to motivate adherence to lifestyle and other therapies. They can promote enhanced application of guideline-based therapies as well as improved outcomes [7]. The ACC/AHA Cardiovascular Risk Assessment Working Group [1] felt the development of a new risk calculator was needed that focused on the broader end point of ASCVD [including coronary heart disease (CHD) death, nonfatal myocardial infarction, and fatal and nonfatal stroke] and also included ethnicities beyond Caucasians. The older Adult Treatment Panel III Framingham risk calculator was limited to prediction of hard CHD events (fatal and nonfatal myocardial infarction) and was based on the predominantly Caucasian population of Framingham, Massachusetts. The new pooled cohort risk calculator that is the foundation of the ACC/AHA cardiovascular risk assessment guideline was developed from four major cohorts: the Atherosclerosis Risk in Communities (ARIC) study, the Cardiovascular Health Study (CHS), the Coronary Artery Risk Development in Young Adults (CARDIA) study, and the Framingham Original and Offspring cohorts, which at the time of development had at least 10 years’ follow-up of participants. The risk calculator determines both the 10-year ASCVD risk (among those aged 40–74 years) and the lifetime ASCVD risk (among those aged 20–59 years) and is downloadable onto most smartphones, tablet computers, and personal computers (Figure 1). If there is uncertainty regarding treatment decisions based on the initial risk assessment using the pooled cohort risk calculator, the guideline also recommends assessment of other measures (see “ACC/AHA 2014 Guideline for Cholesterol Management”) to further inform treatment decision making.

The 2013 ASCVD Risk Estimator.
ASCVD, atherosclerotic cardiovascular disease. From the American College of Cardiology (ACC)/American Heart Association (AHA) risk assessment guideline [1].
Lifestyle management remains the cornerstone of preventive cardiology, including cholesterol management. The principal recommendation of the ACC/AHA lifestyle management guideline [2] is for adults who would benefit from LDL cholesterol (LDL-C) level or blood pressure lowering, to have a dietary pattern emphasizing vegetables, fruits, and whole grains, low-fat dairy products, poultry, fish, legumes, nontropical vegetable oils and nuts, and limiting intake of sweets, sugar-sweetened beverages, and red meats (class I, level of evidence A recommendation). A favorable dietary pattern also achieves 5–6% of calories from saturated fat, with a reduction in calories from trans fats and consumption of no more than 2400 mg of sodium daily (or at least a reduction of sodium intake of at least 1000 mg per day). Moderate to vigorous aerobic physical activity is recommended three or four times per week for approximately 40 min per session. For overweight and obese individuals, the ACC/AHA/The Obesity Society guideline for the management of overweight and obesity in adults [3] notes that even modest weight loss of 3–5% of body weight can result in clinically meaningful reductions in triglyceride, blood glucose, and glycated hemoglobin levels, and prevention of development of type 2 diabetes. Central to this guideline is the advice that (1) overweight and obese individuals participate for at least 6 months in a comprehensive lifestyle program, adhering to a reduced-calorie diet and increased physical activity, and (2) high-intensity (≥14 sessions in 6 months) comprehensive weight loss interventions prescribed by a trained professional (e.g. dietitian or exercise physiologist).
ACC/AHA 2014 Guideline for Cholesterol Management
This guideline [4] focuses on (1) identification of four statin benefit groups for ASCVD risk reduction, (2) a new perspective on the use of LDL-C goals, (3) emphasis on the clinician-patient risk discussion, (4) use of global risk assessment for primary prevention, and (5) safety recommendations.
Four statin benefit groups were defined and included those individuals with (1) clinical ASCVD, (2) an LDL-C level of 190 mg/dL or greater and aged 21 years or older, (3) diabetes and aged 40–75 years with an LDL-C level of 70–189 mg/dL, and (4) primary prevention without diabetes with a 7.5% or greater 10-year ASCVD risk, aged 40–75 years, and an LDL-C level of 70–189 mg/dL (Figure 2). In patients in each of these groups, depending on age and risk level, either moderate-intensity statin therapy to reduce the LDL-C level by 30% to less than 50% from the baseline or high-intensity statin therapy intended to reduce the LDL-C level by 50% or more from the baseline is indicated. Moreover, in primary prevention, there is also consideration for moderate-intensity statin therapy even in those at lower risk (5 to >7.5% 10-year risk). These are now the intended “therapeutic goals” rather than the use of specific LDL-C goals (e.g. >70 mg/dL for very high risk persons) since the guideline committee determined that there was a lack of randomized clinical trial evidence to support titration of drug therapy to specific LDL-C and/or non–HDL cholesterol goals. However, given the wealth of clinical trial data on higher-intensity versus lower-intensity statin therapy [8], there was strong evidence that the appropriate intensity of statin therapy should be used to reduce ASCVD risk in those likeliest to benefit. Therefore the guideline took the bold step of abandoning specific LDL-C goal levels that have been the principal therapeutic target in lipid management for decades. In addition, there is emphasis on evaluation of net clinical benefit, in which potential harm must be weighed against potential benefits. Of interest, the cutpoint of 7.5% or greater for consideration of statin therapy in primary prevention is consistent with the level of risk where the number needed to treat to prevent an ASCVD event is favorable compared with the number needed to harm based on the projected incidence of statin side effects (most of which are incident diabetes, despite its relatively low rate and somewhat arbitrary definition).

Summary of Statin Initiation Recommendations for the Management of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Disease (ASCVD) Risk in Adults [4].
ABI, ankle brachial index; ACC, American College of Cardiology; AHA, American Heart Association; CAC, coronary artery calcium; DM, diabetes mellitus; hs-CRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol; y, years.
While the statin-eligible groups mentioned above indicate where the evidence is clear regarding those who would benefit from statin therapy for ASCVD risk reduction on the basis of clinical trial data, it is emphasized that these guidelines are not a “point and shoot” approach, but one that is based on conducting a careful clinician-patient discussion before starting statin therapy, especially in primary prevention. This includes discussing with patients their estimated 10-year ASCVD risk (in those without established ASCVD who are by definition high risk) and reviewing other risk factors and strategies for their control, including the potential for benefit from a heart-healthy lifestyle and consideration of referral to a dietitian and/or exercise physiologist. Further, the potential benefit versus adverse effects of therapy should always be discussed, as should patient preferences. These concepts are paramount to the strategy of shared decision making in which the patient is an equal partner in decisions regarding appropriate care rather than a one-sided “doctor prescribes and tells the patient what to do” approach. Strategies for shared decision making are an important focus of recently released guidance from the ACC/AHA in lipid management [9].
For patients in the statin-eligible groups and those not explicitly in these groups (e.g. those younger than 40 years or older than 75 years with diabetes or candidates for primary prevention) when treatment is uncertain, the guideline indicates specific factors that may inform the decision. These include a family history of premature ASCVD, elevated lifetime risk of ASCVD, an LDL-C level of 160 mg/dL or greater, a high-sensitivity C-reactive protein level of 2.0 mg/dL or greater, a coronary artery calcium score of 300 or greater than or equal to the 75% percentile for age, gender, and ethnicity, and an ankle brachial index of less than 0.9. The influence of this information on management decisions requires discussion between the clinician and the patient. Finally, while specific LDL-C targets were removed from the ACC/AHA guideline, this document continues to (1) emphasize adherence to medication and lifestyle and (2) assessment of therapeutic response to statin therapy and safety. These measures require the monitoring of the fasting lipid panel (4–12 weeks after initiation of therapy and every 3–12 months thereafter) to monitor the therapeutic response. Safety laboratory data should be obtained as clinically indicated.
Recommendations for Consideration of Nonstatin Therapies
Importantly, the ACC/AHA cholesterol management guideline indicates that in those at higher ASCVD risk receiving the maximum tolerated intensity of statin therapy (which may be no therapy in a statin-intolerant individual) that the addition of a nonstatin cholesterol-lowering drug with proven efficacy may be considered if the ASCVD risk reduction benefits outweigh the potential for adverse effects [10]. Further guidance with regard to this effect is provided by the ACC/AHA 2016 expert consensus decision pathway on the role of nonstatin therapies [11] in LDL-C level lowering as an update to the 2014 guideline statement. This statement notes that nonstatin therapies (ezetimibe first, PCSK9 mAb second) may be used in select high-risk patients if at least a 50% LDL-C level reduction is not achieved with maximally tolerated statin therapy. These therapies may also serve as alternatives for those individuals who despite maximum tolerated statin therapy are still with (1) an LDL-C level of greater than or equal to 70 mg/dL with ASCVD and other comorbidities, (2) an LDL-C level of greater than or equal to 100 mg/dL with ASCVD but without comorbidities, or (3) without ASCVD but with an initial LDL-C level of 190 mg/dL or greater that is still 70 mg/dl or greater. For patients with diabetes (without ASCVD) or in primary prevention patients with a 10-year ASCVD risk of 7.5% or greater, additional therapies may include ezetimibe therapy followed by use of a bile acid sequestrant if 50% or greater LDL-C level lowering with maximal statin therapy or an LDL-C level of less than 100 mg/dL is not achieved. Moreover, part 2 of the recently released National Lipid Association recommendations [12] also provides guidance for considering the use of PCSK9 mAb therapy, specifically indicating its use when LDL-C targets of less than 100 mg/dL in those with ASCVD or less than 130 mg/dL in those with familial hypercholesterolemia (FH) are not reached. In addition, consideration could also be given to those high risk ASCVD patients (e.g., with recurrent events) whose LDL-C still remains at 70 mg/dl or above despite statin therapy. We recently reported from statin-treated US adults in the National Health and Nutrition Examination Survey (NHANES) 2009–2010 that only 27% of those with CHD had an LDL-C level of less than 70 mg/dL and those not at goal averaged 34 mg/dL above this cutpoint [13]. While it is unclear how many of these patients were receiving recommended moderate-intensity or high-intensity therapy, these data do suggest a significant opportunity for consideration of newer therapies, such as PCSK9 mAb therapy, when reasonable targets cannot be reached.
The IMPROVE-IT Trial and Implications for Cholesterol Management
The recent results of the IMPROVE-IT trial [14] in acute coronary syndrome patients with the addition of ezetimibe therapy confirm the value of additional LDL-C lowering with nonstatin therapy beyond statin therapy. The benefits seen after 7 years of accrual included a significant (albeit modest) 6% relative risk reduction (hazard ratio 0.94, P=0.016) and a number needed to treat of 50 for the primary end point of CVD death, myocardial infarction, hospital admission for unstable angina, coronary revascularization, or stroke (Figure 3). The patients in this trial were very high risk acute coronary syndrome patients randomized shortly (within 10 days) after the onset of acute coronary syndrome, and many of the events occurred within the first year of the trial. Thus the results and benefit of ezetimibe therapy may be limited to high-risk recent acute coronary syndrome patients. While ezetimibe offers an additional 15–20% LDL-C level reduction (the on-trial LDL-C level in IMPROVE-IT was 53 mg/dL in those receiving ezetimibe vs. 70 mg/dL in the placebo group), those with greater LDL-C level elevations despite statin therapy may need additional therapy beyond ezetimibe therapy.
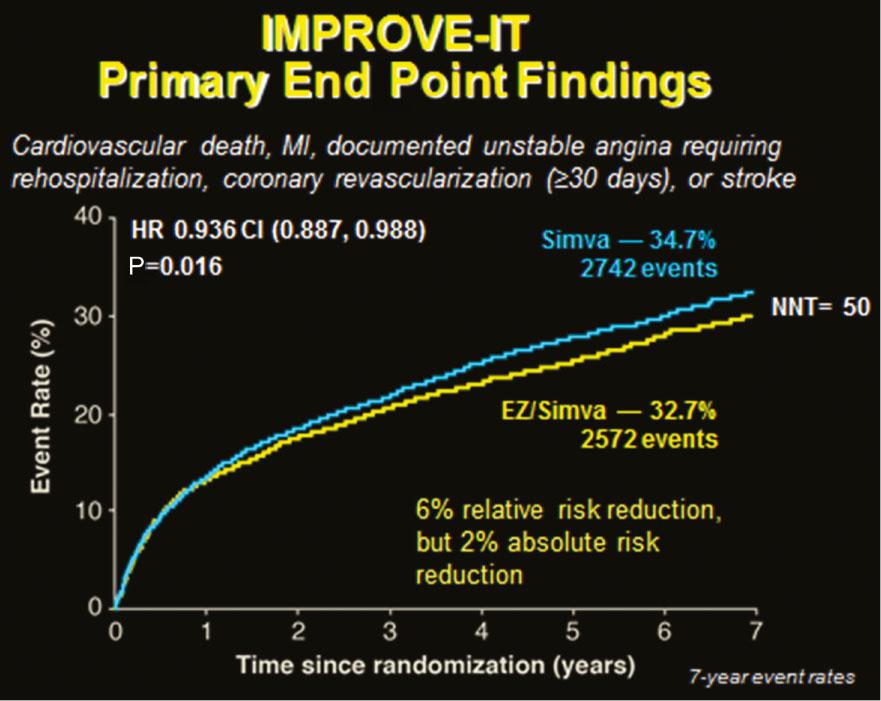
Kaplan-Meier Curve of Cumulative Cardiovascular Disease Event Rates in the IMPROVE-IT Trial [14].
CI, confidence interval; EZ, ezetimibe; HR, hazard ratio; MI, myocardial infarction; NNT, number needed to treat; Simva, simvastatin.
PCSK9 mAb Therapy for Dyslipidemia
Among the most significant advances in cardiology in the past decade is the development of PCSK9 mAb therapy. Alirocumab (Praluent®) and evolocumab (Repatha®) were approved by the US Food and Drug Administration in July–August 2015. In addition, a third PCSK9 mAb product, bococizumab (RN316), is in phase 3 clinical trials. PCSK9 is a 692–amino acid mature protein mainly expressed as a secreted protease in the liver, intestines, and kidneys. This molecule forms a complex with the hepatic LDL receptor, which undergoes endocytosis and destruction of the LDL receptor complex. This process reduces the number of LDL receptors able to process LDL, thereby resulting in increased levels of circulating plasma LDL-C particles and an increased atherogenic state [10, 15]. PCSK9 mAbs bind to PCSK9, which prevents the association of PCSK9 and the LDL receptor. This action inhibits the effects of PCSK9, maintains the LDL receptors, and results in dramatic reductions in LDL-C levels. Multiple phase 2 and phase 3 trials that have examined the efficacy and safety of alirocumab, evolocumab, and bococizumab have shown LDL-C level reductions averaging 50–60% in statin-treated or statin-intolerant patients with or without documented ASCVD. Observed effects on lipid fractions include a 25–39% decrease in LDL-C levels in patients with homozygous FH, an approximately 50% reduction in non–HDL cholesterol and apolipoprotein B levels, and a 25% lowering of lipoprotein (a) levels [15].
Pooled data from relatively short-term safety and efficacy open-label studies were published in spring 2015, providing significant additional insight into safety and preliminary outcomes of treatment with PCSK9 mAbs. The Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with their Lipid Modifying Therapy (ODYSSEY LONG-TERM) placebo-controlled trial [16] evaluated 2341 patients with hyperlipidemia receiving maximally tolerated statins who were at high risk of CHD (69% with prior CHD and 35% with diabetes). Alirocumab (150 mg biweekly) reduced the LDL-C level by 62% at 24 weeks compared with placebo; the mean LDL-C level was 48 mg/dL in the alirocumab group compared with 119 mg/dL in placebo patients. Among the alirocumab group, 79% achieved an LDL-C level of less than 70 mg/dL at week 24, compared with only 8% in the placebo group. Certain adverse events were more frequent in the alirocumab group than in the placebo group: injection site reactions 5.9% versus 4.2%, myalgia 5.4% versus 2.9%, neurocognitive events 1.2% versus 0.5%, and ophthalmologic events 2.9% versus 1.9%. Of particular interest, the post hoc analysis of the composite of cardiovascular events over 78 weeks – including CHD death, myocardial infarction, ischemic stroke, and unstable angina requiring hospitalization – showed those in the alirocumab group compared with those in the placebo group had a 48% reduced risk of such events (1.7% vs. 3.3%, hazard ratio 0.52, 95% confidence interval 0.31–0.60) (Figure 4A). A similar study of evolocumab [Open-Label Study of Long-Term Evaluation Against LDL-Cholesterol (OSLER)] [17] was reported. It included a prespecified combined analysis of 4465 patients who completed 1 of 12 phase 2 or phase 3 studies of evolocumab. The participants were randomized to receive either evolocumab at 420 mg every 4 weeks plus standard of care or placebo with standard of care alone in an open-label extension study averaging 11 months. The evolocumab group showed a 61% reduction in LDL-C levels from 120 to 48 mg/dL (a 72 mg/dL between-group LDL-C level difference) at 12 weeks. There was no difference in the rate of serious adverse events (7.5% in each group). The OSLER study reported a 53% reduction in the incidence of the prespecified composite end point of death, myocardial infarction, hospitalization for unstable angina, coronary revascularization, stroke, transient ischemic attack, and hospitalization for heart failure (0.95% vs. 2.18%, hazard ratio 0.47, 95% confidence interval 0.28–0.78). This can be considered a promising outcome in a short time despite a limited number of events (n=60) (Figure 4B). The reductions in cardiovascular outcomes are very consistent between the two mAbs. If these reductions persist to 2 years, this would suggest a number needed to treat of approximately 50. Large phase 3 trials involving more than 70,000 patients will provide definitive data on reduction in cardiovascular outcomes; the first of these is due for completion by early 2017.

Cumulative Cardiovascular Disease Event Rates in the (A) ODYSSEY OUTCOMES [16] and (B) OSLER 1 and OSLER 2 [17] trials.
CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; LLT, lipid-lowering therapy; MI, myocardial infarction; pts, patients; RRR, relative risk reduction; TEAE, treatment-emergent adverse event; TIA, transient ischemic attack.
The current indications for both alirocumab and evolocumab, as approved by the Food and Drug Administration, involve their use as adjuncts to diet and maximally tolerated statin therapy for adults with heterozygous FH or clinical ASCVD who require additional LDL-C level lowering [11, 12]. Evolocumab is also indicated for such individuals with homozygous FH who require additional LDL-C lowering [18, 19], including use by adults and adolescents aged 12 years or older with homozygous FH in combination with other lipid-lowering therapies. However, both products clearly state in their labeling that the effects on cardiovascular outcomes have not been determined.
Conclusions
The ACC/AHA guideline for blood cholesterol management focuses on the identification of four major statin-eligible groups and has as its foundation appropriate ASCVD risk assessment for appropriate targeting of therapy in primary prevention. It also promotes appropriate lifestyle and treatment of obesity as the basis of preventive cardiology and lipid management. While many patients will achieve an adequate therapeutic response from the prescription of moderate-intensity or high-intensity statin therapy, some patients, particularly those who cannot tolerate statins or who have a very high baseline LDL-C level (e.g. FH patients) will require the addition of nonstatin therapy. The IMPROVE-IT trial suggests a potential role for cholesterol absorption inhibitor therapy in combination with a statin in patients with acute coronary syndrome, whereas the remarkable LDL-C level lowering achievable by PCSK9 mAb is potentially a valuable approach to further address residual ASCVD risk. Confirmation of this strategy will depend on the results of large-scale trials of the unique class of PCSK9 mAb currently in progress.
