Introduction
Long-term survival for patients with congenital heart disease (CHD) has dramatically improved thanks to tremendous advances and improvements in pediatric and young adult care. A significant population of adults with CHD now exists, with adults outnumbering children with CHD, as well as a rising median age of adult survivors with severe forms of CHD [1–3]. Pediatric patients with CHD are expected to survive to adulthood, and adult CHD (ACHD) management has even embraced geriatric care issues for older patients with CHD. Arrhythmias remain a significant long-term complication for CHD survivors, impacting both morbidity and mortality. Furthermore, arrhythmias such as atrial fibrillation (AF), which previously were not commonly tied to CHD, are increasing in prevalence among CHD survivors because of the natural epidemiology of the arrhythmia itself as well as the evolving sequelae in an aging ACHD population.
Mechanisms and Substrates for Atrial Arrhythmias in CHD
AF in the setting of CHD is epidemiologically and mechanistically different from that encountered in the general population. The arrhythmia occurs at younger ages in patients with CHD, where it is typically observed in the setting of organized atrial arrhythmias that degenerate to AF, and can progress rapidly to persistent or permanent forms of AF [4–6]. More than half of patients with severe CHD who reach the age of 18 years have atrial arrhythmias by the age of 65 years, and have a significant increase in morbidity and mortality compared with those ACHD patients without atrial arrhythmias [7].
A spectrum of CHD substrates exists, contributing to a substantial heterogeneity in the development and presentation of atrial tachyarrhythmias in CHD (Figure 1). Structural disease in ACHD extends from single to multiple anomalies and exists in the native unoperated on or procedurally/surgically palliated or corrected states. Palliated and corrected forms of CHD differ on the basis of the specific cardiovascular substrate and the specific procedural/surgical techniques used in different eras of management. These then contribute to great differences with regard to anatomic and pathophysiologic sequelae following previous interventions. Common sequelae in adults with previous surgery include pulmonary regurgitation in the setting of previous repair of dysfunctional right ventricular outflow tracts, development of cardiomyopathy in the setting of congenitally or surgically corrected transposition of the great arteries, and cardiomyopathy following staged palliation among the spectrum of single-ventricle defects. Valvular and conduit dysfunction, ventricular dysfunction, and hemodynamic abnormalities result in secondary electrophysiologic changes in atrial and ventricular myocardium. Furthermore, incisional scars, suture lines, patch materials, and myocardial fibrosis create substrates that support a host of electrophysiologic abnormalities.

Structural, Anatomic, Electrophysiologic, and Hemodynamic Contributors to the Progressive Risk and Development of Atrial Arrhythmias in Patients with CHD.
The potential substrates and mechanisms of AF initiation and perpetuation are multiple, complex, and incompletely elucidated. The development of a primary atrial myopathic process in CHD has many potential causes, including abnormal atrial morphologic, histologic, pathophysiologic, and electrophysiologic features. The interplay of factors includes atrial myopathic structural changes associated with atrial myocardial hypertrophy, inflammation, and macroscopic and microscopic fibrosis. Histopathologic evidence of atrial fibrosis is typically seen in CHD patients with AF [8]. Total atrial conduction time derived from tissue Doppler imaging in ACHD patients is associated with the development of atrial arrhythmias [9]. In addition to CHD-specific factors, recognized contributors to AF in the structurally normal heart are also likely to be at play to drive the development of AF in CHD. These include factors such as premature atrial contractions and atrial tachycardia triggers emanating from pulmonary vein and atrial foci, degeneration to AF from stabler supraventricular tachycardias (SVT), atrial anatomy and electrophysiology associated with rotors, vagal and sympathetic autonomic influences, activation of the renin-angiotensin-aldosterone system, and significant bradycardia and pauses [10]. The contribution of incisional scars in the atria from prior congenital cardiac surgery to the development of macroreentrant atrial tachyarrhythmias is also a recognized concomitant arrhythmia as well as an arrhythmic contributor to AF in CHD. Postoperative incisional intraatrial reentrant tachycardia (IART) circuits develop in viable atrial myocardium with heterogeneous conduction properties surrounding incisional scars and other fibrous structures, which act as conduction barriers [11]. Finally, ventricular dysfunction, the complexity of CHD anomalies, and increasing age all augment the risk of supraventricular arrhythmias, including AF and atrial flutter, in CHD survivors [12]. The combined impact of incisional scars, abnormal hemodynamics with volume and pressure loading, atrial chamber dilatation and increases in atrial wall thickness and distribution of fibrosis results in a highly dynamic and continuously changing atrial substrate that is highly predisposed to arrhythmias.
The spectrum of atrial arrhythmias that are commonly associated with and can lead to AF include focal atrial tachycardias, typical cavotricuspid isthmus–dependent atrial flutter, and atypical and incisional reentrant atrial flutters [13]. Depending on the specific CHD anatomy and pathophysiology, the atrial arrhythmias can involve the right atrium, the left atrium, or both atria and can encompass single or multiple arrhythmia mechanisms, predominantly with macroentrant and microreentrant mechanisms [14]. From a simplistic perspective, patients with left-sided defects or who have had palliative surgery for CHD are thought to be more prone to develop AF, while IART occurs more commonly in the right atrium [13, 15]. In cases of concomitant AF and IART, ACHD patients with right-sided anomalies had a higher arrhythmia burden over their lifetime than patients with left-sided anomalies, while incidence of stroke, heart failure, and mortality were comparable [16]. In the setting of CHD with right atrial overload, atrial myopathy as evidenced by histologic assessment of structural remodeling and fibrosis is associated with the presence of supraventricular arrhythmias [17].
Macroreentrant atrial arrhythmias are seen across a broad spectrum of CHD types, including atrial septal defects (ASDs), single-ventricle defects, transposition of the great arteries, and tetralogy of Fallot [18]. Given the frequent involvement of the cavotricuspid isthmus in typical atrial flutter in the structurally normal heart, it should not be surprising that it is also frequently involved in IART with CHD. Most IART circuits in CHD patients on electrophysiologic study were cavotricuspid isthmus–dependent macroreentrant circuits, followed by circuits involving incisional scars [19]. Successful catheter ablation was achieved in both of these settings [20]. Furthermore, cavotricuspid isthmus–dependent flutter and scar-associated macroreentry often occur in the same patient, warranting careful testing and mapping to appropriately differentiate and ablate both substrates [21]. In right-sided CHD, there was a higher incidence of cavotricuspid isthmus–dependent circuits, but a higher incidence of postablation recurrence for non-cavotricuspid isthmus–dependent circuits [22]. Other mechanisms of IART have been described in specific patient subsets, such as pericaval reentry in Fontan patients [23]. Given the high incidence of cavotricuspid isthmus–associated macroreentry, some authors suggest that surgical incisions in the right atrium at the time of repair should be extended to the inferior vena cava as a primary approach to the prevention of IART [24]. While most arrhythmias that occur late after surgical repair of CHD are macroreentrant, some are focal atrial tachycardias that require alternative strategies for ablation [25]. Automaticity is often the mechanism in these cases; however, nonautomatic and microreentrant varieties exist, and can be differentiated on the basis of features of arrhythmia inducibility and termination properties as well as dense and detailed substrate mapping [26]. There are overlapping substrates, which results in both atrial tachycardia and AF. Chronic and persistent atrial tachycardias can cause progressive atrial myopathy that then results in AF development. Primary progressive atrial myopathy manifests itself as IART initially but further progresses to confer increased risk of AF with time.
Given the often compromised hemodynamics in ACHD, the presence of AF can potentially cause symptoms, hemodynamic detriment, or even death. Deleterious hemodynamic effects of atrial arrhythmias during exercise may be significant in ACHD [27]. Clinical variables associated with increased mortality in the setting of atrial arrhythmias and ACHD include poor functional class, single-ventricle physiology, pulmonary hypertension, and valvular heart disease [28]. The association of AF with lethal ventricular arrhythmias is a rare but well-appreciated complication in the setting of Wolff-Parkinson-White syndrome. Wolff-Parkinson-White syndrome is associated with certain forms of CHD and structural heart disease, including Ebstein anomaly and hypertrophic cardiomyopathy, which is likely related to the structural and myocardial abnormalities in the primary conditions themselves giving rise to abnormal, and sometimes multiple, atrioventricular connections [29, 30]. Alternatively, Wolff-Parkinson-White syndrome is rarely an incidental cause of arrhythmias in CHD patients. Among patients with palliated single-ventricle CHD, Correa et al. [31] noted that accessory pathway–mediated SVT occurred in four of 52 patients, with Wolff-Parkinson-White syndrome noted in two patients (3.8%). Neither patient experienced syncope or documented rapidly conducted atrial arrhythmias, and both had pathway refractory periods of 280 ms, which would not be considered to carry concerning risk of ventricular arrhythmia potential in the setting of AF. A recent publication noted no increased risk of death among hypertrophic cardiomyopathy patients with Wolff-Parkinson-White syndrome compared with those without Wolff-Parkinson-White syndrome [32]. In the setting of Wolff-Parkinson-White syndrome, ablation of the accessory pathway is often associated with cure of AF, most likely due to the eradication of reentrant SVTs utilizing the pathway that degenerate to AF [33]. However, this may not be the same in scenarios of AF in CHD, where the development of AF is complex and multifactorial and likely not linked only to the presence of Wolff-Parkinson-White syndrome.
The epidemiology, incidence, and prevalence of the spectrum of atrial arrhythmias, including AF, differ according to specific CHD types, prior surgical or percutaneous treatment, and residual and ongoing structural and functional sequelae. Certain substrates are recognized to be associated with a higher incidence of atrial arrhythmias, including large and late repaired or unrepaired ASDs, Ebstein anomaly, and single-ventricle CHD following Fontan palliation particularly those with atriopulmonary connections [34].
Atrial Arrhythmias in Specific Forms of CHD
While the risk of AF in CHD involves a complex interplay between CHD type, repaired status, acquired histopathologic and electrophysiologic derangements secondary to past surgery, and hemodynamic abnormalities, as well as increasing age and cumulative time of survivorship with CHD, its development and incidence have been studied in specific CHD types. Traditionally, AF has been associated with primary left-sided defects, and particularly those that result in chronically elevated left atrial or pulmonary venous pressures, leading to chamber dilation and wall thickening and fibrosis, along with common AF triggers involving the pulmonary veins. However, the potential for AF theoretically exists across all types of CHD, owing to recognized and relatively conserved risk factors across a broad spectrum of defect types [5].
In the setting of ASDs, longer times before defect closure appear to be strongly associated with the development of atrial arrhythmias in general, including AF. Age at the time of surgical repair greater than 25 years was a predictor of AF [35]. Among adult ASD patients, those with atrial arrhythmias tended to be older and, while the incidence of atrial flutter decreased after surgery, the incidence of AF did not change [36]. The co-association of atrial flutter and AF following ASD repair also appears to involve overlapping mechanisms. In IART associated with ASD repair, cavotricuspid isthmus–dependent atrial flutter was most common, followed by atriotomy-dependent flutter and double loop atrial flutter, respectively. Owing to the substantial changes to the atrial myocardium in the setting of a long-standing atrial-level shunt as well as those incurred following surgical repair, the morphology of the flutter waves on surface ECG may appear atypical even in cavotricuspid isthmus–dependent circuits [37]. The mechanism did not depend on whether the repair was performed with a primary suture or a patch repair approach. AF in these settings appears to involve both right and left atrial substrates, with right atrial reentrant and focal activation from the right atrium in patients with paroxysmal AF, and multifocal activation from pulmonary veins and the posterior left atrium as well reentrant left atrial mechanisms in patients with persistent AF [38].
Atrial tachyarrhythmias, including AF, are the most common long-term sequelae among survivors of single-ventricle palliation. The presence of atrial tachyarrhythmias was a strong predictor of morbidity and mortality among Fontan patients [39]. Prophylactic atrial arrhythmia surgery to prevent the development of IART in Fontan patients has been proposed along with aggressive arrhythmia surgery at the time of Fontan conversions to reduce the future burden of atrial arrhythmias and their associated impact on long-term morbidity and mortality [40–42].
Cavopulmonary connections culminating in the Fontan operation constitute the current approach to palliation. Since its early development and application in the late 1970s, the Fontan operation has undergone various modifications. Regardless of the form of the Fontan operation, atrial arrhythmias remain a common long-term problem, particularly as patients continue to age and because of a dynamic and progressive atrial myopathic process in these patients [43]. While arrhythmia incidence is highest among classic atriopulmonary Fontan patients, this may be less predominantly related to the Fontan operation type, with a larger contribution of longer duration of follow-up and sequelae related to long-standing hemodynamic abnormalities and single-ventricle dysfunction [44–46].
Lateral tunnel and extracardiac Fontan operation types have a perceived advantage with regard to arrhythmia risk, attributed to less atrial dissection, less long-term atrial chamber enlargement and distortion, and improved mechanical functionality of the Fontan conduit (Figure 2). However, a truly comparable cohort of lateral tunnel or extracardiac conduit variants is lacking in terms of the duration of follow-up in comparison with classic Fontan patients. In adult Fontan patients, IART appears to be the most common atrial arrhythmia, followed by combined IART and AF, and then AF alone, despite the evolution of surgical approaches to the Fontan procedure [47]. The onset of arrhythmias also occurs fairly quickly following the Fontan procedure, with 50% of patients developing atrial tachycardia or AF within 10 years, increasing to 100% at 26 years of follow-up [48]. In a recent single-center experience, up to 40% of Fontan patients had IART by the age of 25 years [49]. Several additional factors also appear to augment arrhythmia risk, including several features that even predate Fontan completion among single-ventricle patients. The presence of significant atrioventricular valve regurgitation, even before Fontan completion, was associated with a higher incidence of atrial flutter [50]. Inducibility of IART was greater in female single-ventricle patients and patients who were older at the time of second-stage palliation or pre-Fontan evaluation [51]. The presence of atrial isomerism appeared to be associated with an increased risk of atrial arrhythmias following Fontan completion [52].
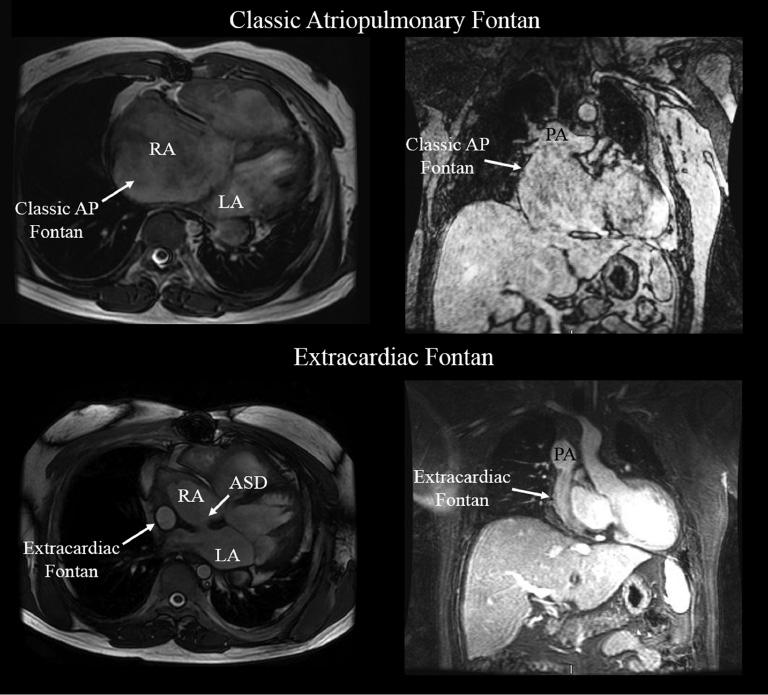
Cardiac MRI Frames Highlighting the Anatomic Differences Between Classic Atriopulmonary and extracardiac Fontan variants.
The drastic difference between the markedly dilated right atrial chamber in the class Fontan compared to the extracardiac conduit is easily seen.
Sinus node dysfunction and the need for atrioventricular valve repair are frequently present among single-ventricle patients with atrial isomerism, and were predictors of future atrial arrhythmias in these patients as well. Assessment of right atrial histopathologic features 7 years after total cavopulmonary surgery demonstrated atrial intimal thickening, smaller myocardial cell size, and interstitial fibrosis [53]. These chronic and progressive changes create atrial substrates with altered electrophysiologic properties with variable conduction times and refractoriness. The finding of significantly prolonged P wave duration Fontan patients with atrial arrhythmias compared with those without them is evidence of this histopathologic-electrophysiologic link [54, 55].
In tetralogy of Fallot, ventricular arrhythmias and sudden death risk are frequently discussed, but atrial arrhythmias, including AF, are more commonly seen. Chronic right ventricular dysfunction related to outflow and pulmonary arterial stenosis and/or pulmonary regurgitation is considered a prominent contributor to right atrial dysfunction. Elevations in right ventricular filling pressures and the presence of significant tricuspid insufficiency can contribute to right atrial chamber dilation and histopathologic changes in response to these chronic hemodynamic derangements. In addition, atrial incision and dissection at the time of surgical repair further contributes to a primary atrial myopathic process [56–60]. The presence of significant left ventricular diastolic dysfunction is recognized as a risk factor for ventricular arrhythmias and sudden death, and may be a contributor to left atrial dysfunction as well [61, 62].
Among patients with repaired transposition of the great arteries, atrial arrhythmias are most frequently encountered in patients who have undergone atrial switch operations. Extensive atrial dissection, suture lines, and subsequent scarring and fibrosis are considered the dominant contributors to the dysfunctional atrial substrate that is prone to both tachyarrhythmias and bradyarrhythmias. In addition, the chronic hemodynamic perturbations secondary to systemic right ventricular dysfunction result in altered and increased atrial baffle pressures, chamber dilatation, and histopathologic changes. The incidence of atrial tachyarrhythmias following atrial switch operations has been reported to be as high as 34% with long-term follow-up, and even up to nearly 50% in some single-center longitudinal studies [63, 64]. The risk of IART appears to differ depending on the type of atrial switch operation, with the risk being 16% in Mustard patients versus 6.1% in Senning patients in published long-term follow-up experience [65]. Regardless of the operation type, the development of IART was associated with worse prognosis. Furthermore, the development of IART in this form of repaired CHD is associated with abnormal atrial and ventricular electromechanical function and risk of sudden cardiac death, considered in part to be due to rapid atrioventricular nodal conduction of atrial tachyarrhythmias resulting in ventricular arrhythmias [66–68].
Eisenmenger syndrome highlights a unique combination and culmination of contributors to atrial tachyarrhythmia development. Chronic shunting typically in the setting of unrepaired CHD as well as the development of pulmonary vascular disease and pulmonary hypertension likely results in gross atrial chamber changes along with histopathologic and electrophysiologic changes due to the long-standing and ongoing hemodynamic disarray. A recent review of arrhythmias in this population of patients showed that nearly one-fifth of patients experienced atrial and ventricular arrhythmias, with atrial arrhythmias occurring more frequently [69]. Of the atrial arrhythmias documented, AF accounted for 50%. The presence of atrioventricular valve regurgitation and QRS prolongation and the absence of Down syndrome were associated with increased arrhythmia risk, and history of arrhythmias and history of antiarrhythmic drug therapy were both predictors of increased mortality.
For all patients with AF, there is no simple or single algorithm for management regarding rate and rhythm control and stroke prophylaxis. This is particularly true for the CHD population because of the great number of factors that need to be taken into consideration, including the specifics of the anatomy and pathophysiology of the specific CHD as well as the sequelae of palliative or reparative therapies and surgical procedures. With complete and specific knowledge about an individual patient’s CHD history, a therapeutic plan can be achieved that is based on symptoms, chronicity (new onset, paroxysmal, persistent, chronic), rhythms involved (atrial tachycardia, atrial flutter, AF), cardiac structure and function, ventricular rate response in AF, sinus and atrioventricular nodal function, limitations to pharmacologic therapy due to renal and hepatic function, and risk of thromboembolic events. The variety of treatment options is defined through the filter of these factors in the individual patient.
Rate and Rhythm Control Strategies for Atrial Arrhythmias in CHD
Pharmacologic Therapy
Given the multiple substrates, mechanisms, and triggers for atrial arrhythmias, including AF, in the CHD population, pharmacologic therapy is still a mainstay of treatment. Guidelines for pharmacologic therapy for AF exist, with exclusion or very cautious use of antiarrhythmic medications on the basis of multiple structural, functional, electrophysiologic, metabolic and genetic factors [10]. In the setting of CHD, many pharmacologic agents may be contraindicated because of the increased risk of proarrhythmia in the setting of structural heart disease and reduced ventricular function. Furthermore, these agents may exacerbate concomitant sick sinus syndrome, and dosing may be further limited by coexisting disease processes affecting renal and hepatic function that impact drug metabolism and excretion. The agents used for primary ventricular rate control, such as calcium channel blockers and beta blockers, may be problematic in patients with depressed ventricular function.
Pharmacologic rhythm control strategies in CHD patients with atrial arrhythmias have evaluated the efficacy of class I and class III antiarrhythmic agents with various degrees of success. Owing to their proarrhythmic potential, particularly in the setting of myocardial scar, baseline conduction abnormalities, and systemic ventricular dysfunction, class IC agents are used with great caution given the frequent presence of these features in CHD patients with arrhythmias. Class III agents, including amiodarone, sotalol, and dofetilide, have also been used. Sotalol is effective in children with operated on CHD although it can unmask sick sinus syndrome requiring pacing [70]. It was also effective for single-dose pharmacologic cardioversion in adolescents and adults with CHD, but again can unmask coexisting sick sinus syndrome [71]. Dofetilide does not appear to precipitate sick sinus syndrome but carries a risk of QT prolongation, ventricular proarrhythmia, and torsade de pointes [72]. The agent was recently studied in a multicenter prospective study evaluating its efficacy and side/adverse effects profile in ACHD patients with atrial arrhythmias [73]. While it was initially successful for rhythm control in nearly 70% of patients, and partially successful in the remainder, the long-term use rate was only 49% at 3 years, with many patients stopping using the medication because of reduced efficacy and side effects. Finally, amiodarone is widely used and generally accepted as an effective short-term and long-term antiarrhythmic agent for the treatment of atrial arrhythmias with less concern about cardiac-specific adverse effects even in patients with reduced ventricular function. Hypotension and bradycardia were common side effects during short-term intravenous administration among pediatric patients treated for various forms of SVT [74]. However, its long-term use can be problematic, particularly in younger patients, because of potential multiorgan toxic effects.
Invasive Therapies
Definitive treatment of atrial arrhythmias, including AF, in CHD remains limited to mechanical disruption of the tissue substrate responsible for arrhythmia initiation and propagation. This includes catheter-based ablative modification within the atrial chambers or surgical therapies that create physical barriers to electrical conduction (i.e., incisions and suture lines) or resect highly diseased and arrhythmia-prone tissue or portions of the chamber to result in size reduction. The specific interventional approach to treating these arrhythmias depends on a thorough understanding of the complex interactions between specific CHD anatomy and pathophysiology of the CHD, previous surgical operations and percutaneous procedures, resultant histopathologic and electrophysiologic changes over time, and the specific arrhythmias that have been documented.
Advanced Cardiac Imaging for Procedural Planning
Advanced imaging with CT or MRI can provide valuable information for the evaluation, planning, and decision making processes for arrhythmia treatment in the broad spectrum of young and older CHD patients with high-burden or highly symptomatic arrhythmias. Recent advances in CT techniques now permit highly detailed imaging in pediatric and young adult CHD patients, with submillisievert studies achievable in infants and young children. Alternatively, cardiac MRI permits detailed imaging without ionizing radiation exposure, but can be limited in cases involving non–magnetic resonance conditional cardiac rhythm devices and in patients with renal insufficiency, preventing administration of gadolinium-based contrast agents. With both imaging modalities, artifacts due to sternotomy wires, surgical clips, and prosthetic valves can be problematic.
Both CT and MRI provide high-resolution imaging, not only for delineating general anatomy, chamber size and orientation, and proximity of structures to each other, but also for tissue characterization, which can provide an understanding of arrhythmia mechanisms and potential targets for treatment. Of particular relevance in the treatment of atrial arrhythmias in CHD, advanced imaging can characterize the degree of atrial myopathy with regard to atrial size, shape, and function and the degree of macrofibrosis and microfibrosis. Atrial volume and function can be important for decision making to proceed with AF ablation, as larger atria are associated with a higher recurrence rate of AF [75]. The distribution and extent of atrial fibrosis based on cardiac MRI delayed contrast enhancement can provide valuable information regarding potential macroreentrant and microreentrant circuits and other substrate that may promote focal tachycardia or AF. The atrial septum can be clearly visualized with regard to the presence of a patent foramen ovale, ASD, or previous ASD patch or device, as well as its orientation and lie, which may influence transseptal puncture for pulmonary venous atrial access [76]. The presence, patency, and integrity of interatrial baffles can be characterized. Advanced imaging also clearly defines pulmonary venous anatomy, the relation of the atria to thoracic structures such as the esophagus, bronchi, and aorta, coronary venous anatomy, and systemic venous drainage patterns, including the presence or absence of left-sided superior vena cava and inferior vena cava interruption. These imaging tools can also provide valuable information regarding chamber size, ventricular function, and valve function. Both CT and MRI can also demonstrate other non-cardiac congenital abnormalities in the chest and abdomen [77, 78]. Finally, advanced imaging provides clear delineation of baffle and conduit structures, which may be the sites of arrhythmia foci or anatomic complexities that may influence the approach to percutaneous arrhythmia therapeutic interventions (Figure 3, Video 1 [Video 1 is available at the following link https://youtu.be/H16F7zHK4Q4]).
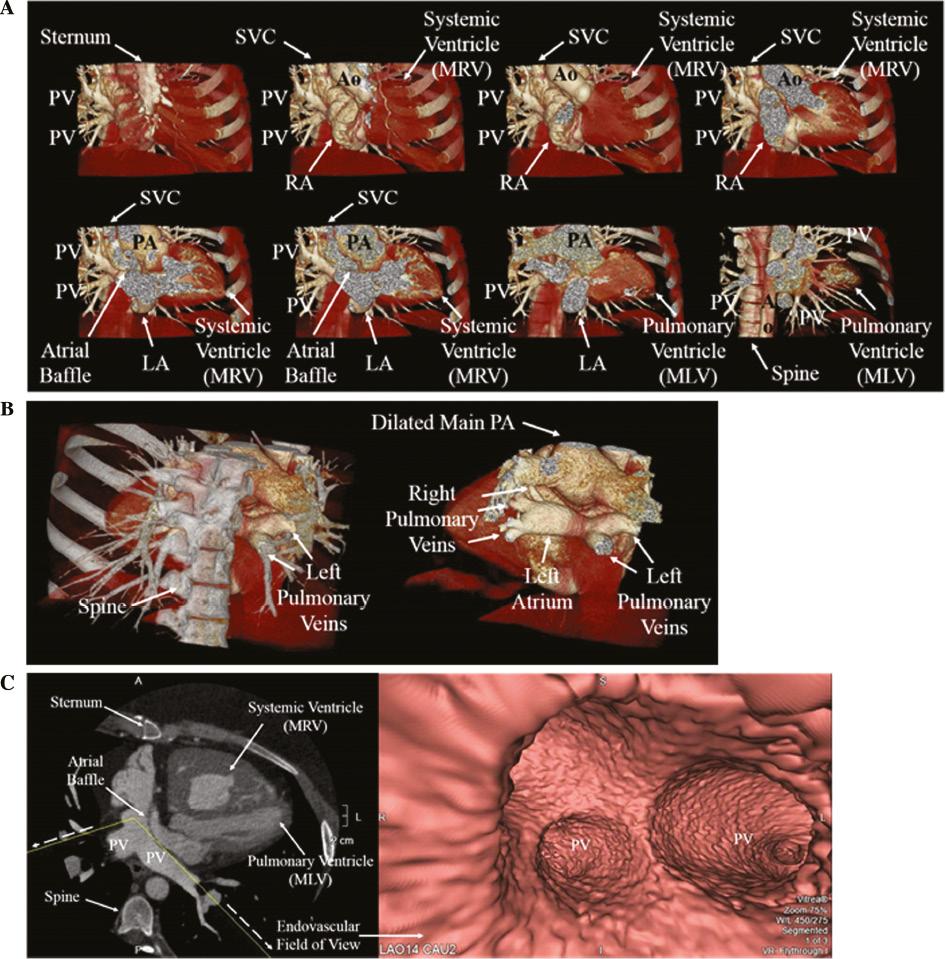
Pre-Ablation Planning Cardiovascular Computed Tomographic Images for an Adult Patient with D-Transposition of the Great Arteries Status Post Mustard Atrial Baffle in Childhood with a History of IART.
(A) Coronal 3-D anterior to posterior images demonstrating D-Transposition of the Great Arteries status post Mustard atrial baffle. LA, left atrium; MLV, morphologic left ventricle; MRV, morphologic right ventricle; PV, pulmonary vein; RA, right atrium; SVC, superior vena cava. (B) Coronal posterior 3-D view demonstrating the pulmonary veins entering the left atrium. Left Panel: Skeletal structure included for orientation. Right Panel: Skeletal structure removed. PA, pulmonary artery. (C) Left Panel: Double oblique 2-D view demonstrating the atrial baffle. Dashed arrows and yellow lines represent the field of view for endovascular images. Right Panel: Endovascular view demonstrating pulmonary veins entering the atrial baffle. MLV, morphologic left ventricle; MRV, morphologic right ventricle; PV, pulmonary vein.
Specific to imaging of Fontan circuits, delayed images or simultaneous contrast injections from upper and lower extremity venous sites are often required to fully delineate the baffle location, size, and course. The datasets from advanced cardiac imaging can also be used for three-dimensional printing of models for procedural planning and virtual computer modeling of macroreentrant circuits and prediction of potential lesion sets. On the basis of these findings, decisions can be made as to whether to proceed with invasive treatments, and if so, whether surgical, minimally invasive, percutaneous, or hybrid procedures are most suitable.
Catheter-Based Therapies
Percutaneous catheter ablation has become the mainstay in definitive arrhythmia treatment in patients with CHD. Both radiofrequency ablation and cryotherapy are used with high degrees of success. While the predominant experience in ablation for atrial arrhythmias in CHD has centered on ablative treatment of IART, focal tachycardias, and conventional SVT substrates, contemporary experience in the management of AF with catheter ablation is slowly growing. Furthermore, given the likely substantial overlap in diseased atrial substrate that contributes to both reentrant atrial arrhythmias and AF in CHD, the current approaches to ablative substrate modification in CHD should target and address both arrhythmia types if they occur in the same individual. As such, the authors advocate a comprehensive ablation approach to address both AF and other arrhythmia types during a single procedure in patients with clearly and independently documented atrial arrhythmias and AF.
Preprocedural planning is critical for the safe and successful performance of catheter ablation in patients with CHD. A thorough and complete understanding of the original type of CHD, all previous reparative and palliative procedures, current anatomical and structural configurations, and current quantitative functional statuses, including ventricular function and clinical functional capacity, is of highest priority. Clear understanding of the association between clinical arrhythmias and potential arrhythmia substrates will help to guide the diagnostic and therapeutic aspects of the procedure. Integration of advanced imaging with conventional echocardiographic modalities is now commonly done in CHD patients undergoing ablative treatments. As previously mentioned, advanced imaging provides critical information for procedural planning, but can also be integrated into the ablation procedure itself, providing the ability to merge CT or MRI data with three-dimensional electroanatomic mapping (Figure 4).

Advanced Cardiac Imaging Integration with Electroanatomic Mapping for Catheter Ablation Planning and Performance in an Adult with a Fenestrated Lateral Tunnel Fontan and Recurrent Atrial Tachyarrhythmias.
Mapping and catheter ablation of multiple ectopic atrial tachycardias in the lateral tunnel was performed. The entire procedure was completed without the use of fluoroscopy. Left Upper Panel: 3-D cardiac MRI reconstruction. The DICOM image set was imported into the EP mapping system, 3-D reconstructed and segmented to the structures of interest. Middle Upper Panel: Merged 3-D cardiac MRI with catheter-based electroanatomic map allowing for real time mapping, visualization of catheter location, and ablation lesion registration on the patient’s cardiac MRI reconstructed 3-D anatomy. An initial voltage map created in sinus rhythm demonstrates areas of scar (grey) and viable myocardium (purple). The voltage map shows the significantly scarred and electrically quiescent lateral tunnel conduit (owing to the diseased right atrial myocardium and Goretex patch used in creation of the lateral tunnel). Preserved voltages were noted in the remnant right atrial cavity in the pulmonary venous circulation (accessed through the fenestration). Yellow dots represent acquired points during map creation which and used for geometry, voltage, and activation. Right Upper Panel: Activation map of an atrial tachycardia. Left Lower Panel. Activation map of a second atrial tachycardia. Both tachycardias arose in a region of heterogeneous scar. The tachycardias exhibited more of a focal activation map, but were likely microreentrant in mechanism owing to their repeated inducibility and termination with pacing. Middle Lower Panel: Demonstration of the planned ablation targets. Right Lower Panel: Ablation lesion points homogenizing the scar region and effectively eliminated the atrial tachycardias in this shared substrate from which the tachycardias arose.
Appropriate anesthesia support, anticoagulation management, and pharmacotherapy adjustments are extremely important to ensure patient comfort, risk reduction, and clinical stability both during and after the ablation procedure. The catheter approach, vascular access, and catheter positioning must be carefully planned on the basis of the type of arrhythmias being treated, available venous and arterial connections to the heart, and patient and cardiac chamber sizes. Access to the pulmonary venous atrium or chamber is frequently required, and the approach to transseptal or transbaffle access needs to be carefully planned following thorough discussion regarding risks with the patient. The feasibility and safety of transseptal and transbaffle punctures following CHD repair or palliation have been reasonably demonstrated [79–85]. Access to the systemic and pulmonary venous atrial chambers is of significant importance for IART ablation involving macroreentrant circuits using the cavotricuspid isthmus. This area of atrial tissue may span portions of both the systemic venous atrium and the pulmonary venous atrium in patients with transposition of the great arteries following atrial switch operations as well as in patients with atriopulmonary or lateral tunnel Fontan configurations. A retrograde approach through the aortic valve can also be used to position the ablation catheter at the cavotricuspid isthmus in CHD substrates involving a systemic morphologic right ventricle with original atrioventricular concordance [86]. For patients where inferior vena cava–atrial continuity is completely absent, as would be the case in extracardiac Fontan completion, pulmonary venous atrial access is dependent entirely on transconduit puncture, catheter entry through a preexisting fenestration, or direct pulmonary venous atrial chamber access through percutaneous transhepatic or transthoracic entry [87, 88]. Moore et al. [89] reported on the utility of CT angiography, which can reveal more directly accessible portions of the chamber or sites for access that afford the shortest distance to chamber entry in Fontan patients.
Adjunctive imaging with intracardiac echocardiography and CT or MRI merging is evolving as a valuable tool to guide ablation performance, improve procedural safety, and reduce radiation exposure. Intracardiac echocardiography provides direct imaging for the presence of intracardiac thrombi, pulmonary vein stenosis, proximity of structures to the esophagus, and presence or development of pericardial effusion. It also provides real-time visualization during transseptal or transbaffle puncture and during ablation delivery to assess catheter tip contact, ablation lesion depth, and formation of microbubbles during radiofrequency energy delivery [90]. Image acquisition, segmentation, landmark registration, and surface registration allow CT or MRI image integration with electroanatomic mapping software, thereby permitting their use during chamber geometrical and arrhythmia mapping.
Targets for ablation are generally determined by a combination of awareness of frequently associated anatomic locations (including the cavotricuspid isthmus and crista terminalis), careful mapping of macroreentrant circuits and focal sites of arrhythmias, and attention to highly suspicious substrate based on anatomic location, tissue characteristics, and distribution of low voltages, signal fractionation, and late potentials [91–95]. While advances in technology have continually been introduced, successful ablation outcomes remain driven by fundamental techniques of entrainment mapping coupled with electroanatomic mapping [96]. However, recurrence rates of arrhythmias highlight the complex, dynamic, and changing substrate that drives arrhythmia development even after effective ablation therapy in CHD.
The role of the pulmonary veins in CHD-associated AF remains unclear. Because CHD-associated AF is typically not seen in the absence of concomitant atrial myopathy, pulmonary vein isolation alone should not be expected to effectively treat AF even as an initial ablation procedure in the setting of paroxysmal AF. However, it is still important to eliminate the contribution of pulmonary vein triggers, either by direct ablation or by electrical isolation, as part of a comprehensive ablative treatment plan for atrial arrhythmias, including AF, in CHD [97–99].
Novel features in three-dimensional electroanatomic mapping systems are being assessed for their utility in complex arrhythmia ablation, and will require specific investigation in CHD populations [100]. The role of rapid mapping technologies is expanding in ablation procedures for CHD patients. Rapid, high-density mapping technologies have provided the ability to collect many points simultaneously, with the information collected at each point including the activation timing, voltage, and comparison with adjacent points. These systems have the potential to shorten the procedural duration while increasing the resolution of electrophysiologic mapping, both of which can contribute to improved procedural safety and ablation success.
Remote magnetic navigation allows catheter manipulation remotely with use of a control console, and has shown utility in catheter navigation within challenging structures such as baffles. The combination of remote magnetic navigation, three-dimensional image integration, and electroanatomic mapping facilitated safe and feasible ablation even in patients with complex anomalies [101]. For patients with complex disease, remote magnetic navigation also has led to a decrease in fluoroscopy time [102]. The development of real-time MRI during catheter ablation procedures permits magnetic resonance–based visualization of transmurality of ablation lesions with gadolinium and identification of gaps in long linear lesions, both of which can result in arrhythmia recurrences following short-term ablation success [103].
Surgical Therapies
Surgical therapies for atrial arrhythmias, including AF, are typically performed concomitantly with other recommended or indicated surgical interventions in CHD patients. The Maze procedure has evolved significantly, with expansion and modification of lesion sets in both atria as well as the adoption of ablative as opposed to incisional lesions. Furthermore, arrhythmia surgery in CHD frequently involves direct tissue excision for removal of arrhythmia-prone substrate and chamber size reduction. Perhaps the best examples of integrated arrhythmia surgery with other CHD surgery are in Fontan conversion surgery for patients with failing Fontan physiology and high arrhythmia burden [104, 105]. When surgical ablative treatments are considered, decisions regarding intervention in the left or right atrium only, biatrial ablation, or surgical left atrial ablation with either presurgical or postsurgical right-sided percutaneous catheter ablation need to be made. Furthermore, given the high incidence of atrial arrhythmias associated with the resultant substrate after congenital heart surgery, some authors have suggested prophylactic lesion sets be performed at the time of surgery to prevent arrhythmia problems such as incisional reentry [42].
Device Therapies
Cardiac implantable electronic devices are an important adjunctive treatment in CHD patients with atrial arrhythmias. Bradyarrhythmias and tachyarrhythmias frequently coexist in patients with previously repaired or palliated CHD, and many CHD patients will develop primary pacing indications over time [106–108]. Severe bradycardia and pauses related to primary sinus node dysfunction can trigger AF and other atrial arrhythmias. Alternatively, CHD patients can have both sinus node dysfunction and separate atrial myopathic substrate–related arrhythmias, owing to prior CHD surgical interventions. In these instances, pacing therapies either are necessary or can be very helpful in addition to pharmacologic therapy or ablation.
Atrioventricular junction ablation and pacing for AF ventricular rate control is useful in scenarios where pharmacologic therapy is either ineffective in achieving ventricular rate control or limited in use by the substrate or pathophysiology. While a transvenous approach can technically be used across a broad range of CHD defects, nodal ablation can be more challenging in patients with complex atrial baffle structures or Fontan circuits [109].
The choice of the type of cardiac implantable electronic device and the route of implantation must be tailored on the basis of specific anatomy, concomitant arrhythmias, native conduction system status, and ventricular functional status. Single-chamber ventricular pacing can be used in patients in persistent or permanent AF. Preservation of atrioventricular synchrony would be desirable in patients with paroxysmal AF. Primary atrial pacing with minimization of ventricular pacing in patients with intact atrioventricular nodal function is preferable in patients with sinus node dysfunction. Consistent atrial pacing may also reduce atrial arrhythmia burden. The integration of atrial arrhythmia preventive treatments or antitachycardia pacing for termination of arrhythmias should be considered if there is sufficient evidence demonstrating their effectiveness or highly suggesting their utility in individual CHD patients with atrial arrhythmias [110, 111].
While transvenous leads have demonstrated better longevity and function compared with epicardial leads, the choice of lead type in CHD patients depends on patient size, venous continuity with atrial and ventricular chambers, and the presence or absence of intracardiac shunts. The risk of thromboembolic events and complications in the setting of transvenous leads and intracardiac shunt lesions is elevated and is not eliminated with concomitant anticoagulative therapies [112]. Among single-ventricle CHD patients lacking typical venoatrial connections, epicardial leads are most commonly placed when pacing is indicated. However, transvenous leads have been implanted in certain unique scenarios. Transvenous lead implantation within the atrial portion of the lateral tunnel Fontan and ventricular pacing lead implantation via the coronary sinus through the Fontan when coronary sinus-to-Fontan atrium continuity is present have been described [113–116]. Finally, transmural (epicardial to endocardial) atrial lead implantation has been described, with the expectant goal of achieving lead functionality and longevity similar to those of standard transvenous endocardial leads in patients lacking standard venoatrial continuity [117].
Stroke Prophylaxis in CHD Patients with Atrial Arrhythmias
The risk of thromboembolic complications and stroke in the setting of CHD and AF is well recognized but incompletely understood from mechanistic and risk-stratification standpoints. In children and adults with CHD, ischemic and hemorrhagic stroke risk is significantly higher than in the general population [118, 119]. Imaging with three-dimensional transesophageal echocardiography has demonstrated a higher incidence of intracardiac thrombi in atrial flutter/AF patients with CHD compared with patients without CHD [120].
In ACHD with atrial arrhythmias, CHA2DS2-VASc scoring predicted thromboembolic events when applied retrospectively [121]. However, the CHA2DS2-VASc scoring system does not specifically account for CHD and is not directly applicable to all CHD patients, particularly those of much younger age and with AF that is technically nonvalvular but still occurring in an atrial substrate with probable elevated risk of thrombus formation. Additional research is required to devise new approaches to stroke risk stratification and to see whether advances in the long-term management of CHD, including the timing of surgery and interventions to minimize the development of arrhythmias, may reduce this thromboembolic risk [119].
Presently, most CHD practitioners would advocate anticoagulation in CHD patients with documented sustained or recurrent atrial arrhythmias, including AF. Additional considerations include the concomitant presence of erythrocytosis, Eisenmenger syndrome, mechanical valves, and conduits with altered or sluggish blood flow. Warfarin is most frequently used, although prospective data on the use of novel oral anticoagulant agents in CHD is growing.
The applicability and indications for percutaneous atrial appendage occlusion or surgical appendectomy in CHD is an area of interest and future research. Concomitant surgical ligation of the appendage at the time of reoperation for other indications in CHD likely has a valuable role for consideration, and it is unclear if appendage occlusion can have a benefit in stroke risk reduction and anticoagulation measures in patients with CHD and atrial arrhythmias, including AF. In regard to the atrial appendages, congenital anomalies exist, including juxtaposition of the atrial appendages, giant congenital left atrial appendage aneurysm, congenital left atrial appendage herniation, and congenital absence of the left atrial appendage [122–126]. These unique morphologic patterns may affect the risk of appendage-associated thrombus formation, and are not currently amenable to percutaneous appendage occlusion strategies. Certainly in CHD conditions with otherwise normal atrioventricular and ventriculoarterial concordance, the role of the left atrial appendage in AF-associated stroke risk is likely similar to that in structurally normal hearts with AF. The possible role of the right atrial appendage or the appendage structures or remnants in morphologically abnormal or surgically altered atria is not entirely clear, nor are percutaneous occlusion devices indicated at present.
Conclusions
Atrial arrhythmias, including AF, in CHD remain a prominent and growing contributor to morbidity and mortality. The mechanisms and substrates for arrhythmia development and progression to varying degrees of AF involve a highly complex and dynamic combination of numerous factors related to the original CHD type, past treatments, continually evolving procedures, and aging of the ACHD population. Application of pharmacologic and invasive therapeutic options for primary atrial arrhythmia and AF treatment as well as stroke prevention necessitates special considerations when applied to the CHD population and requires greater study in specific subsets of CHD patients.
