Introduction
Cardiac arrest, or cardiac death, is defined as an event characterized by the cessation of cardiac activity and lack of perfusion in the absence of a noncardiac cause [1], and may be categorized as out-of-hospital cardiac arrest (OHCA) or in-hospital cardiac arrest (IHCA). It is most commonly a result of ventricular tachyarrhythmias in patients with coronary or structural heart disease. Pathology and angiographic studies have identified that the precipitating cause is often unstable coronary artery plaque [2–4]; therefore the role of percutaneous coronary intervention (PCI) in improving outcomes following cardiac arrest is of great interest. In this review we will briefly detail the epidemiology of cardiac arrest, discuss the rationale for coronary angiography and PCI after cardiac arrest in selected patients, and explore the current evidence.
The epidemiology of cardiac arrest is difficult to study because of the nature of how cardiac arrest patients are identified and reported. Data are primarily gathered through emergency medical encounters, which underestimate the true burden of cardiac arrest, as not all patients with cardiac arrest receive emergency medical services (EMS). It is estimated that the EMS-assessed incidence of OHCA in adults is 1.4 per 1000 person-years, totaling approximately 347,000 cases annually in the United States [5]. The rate of survival to discharge of patients with OHCA is low, but modestly increased from 10.3 in 2009 to 12% in 2016. The first observed cardiac rhythm is shockable [i.e., ventricular fibrillation (VF) or ventricular tachycardia (VT)] in 20% of cases, and when a shockable rhythm is present, the rate of survival to discharge is significantly higher, at approximately 30%. In comparison, there are estimated to be 209,000 cases of IHCA each year in the United States, with a rate of survival to hospital discharge of approximately 25% [6]. The rate of survival to discharge is more than 40% when the initial rhythm is shockable compared with less than 20% when asystole or pulseless electrical activity is present.
Despite improvements in out-of-hospital resuscitation care, such as increased bystander cardiopulmonary resuscitation (CPR) and public access to automated external defibrillators [5], the rate of survival to discharge remains low and has essentially remained unchanged over the past decade (Figure 1). The primary strategies to improve postresuscitation care include targeted hypothermia and coronary reperfusion, if indicated. Targeted hypothermia has now been established as beneficial by three randomized trials and is recommended for all survivors of cardiac arrest [8]. The rationale for early (usually defined as within 12 h of admission) coronary angiography, and PCI if indicated, is to restore coronary flow and salvage myocardium to improve hemodynamics (and thereby improve cerebral perfusion) and reduce the risk of recurrent cardiac arrest. In autopsy series, acute plaque rupture has been identified in 60–70% of people who died of OHCA [2, 3, 9]. When survivors of OHCA systematically undergo early coronary angiography, 64% of patients are found to have significant coronary artery disease, and 37.5% of patients have acute coronary lesions and receive a diagnosis of an acute myocardial infarction [10]. In an analysis of all survivors of OHCA who underwent early coronary angiography at six centers in the United States, 80% of patients with ST elevation myocardial infarction (STEMI) and 33% of patients without STEMI were found to have a culprit lesion on angiography [11]. Observational data from more than 400,000 survivors of VT/VF arrest from the National Inpatient Sample demonstrated an increase in coronary angiography and PCI during index hospitalization following OHCA from 2009 to 2012, and this was associated with an increase in the rate of survival to discharge compared with those who did not undergo angiography [odds ratio (OR) 6.26, 95% confidence interval (CI) 5.93–6.61] [12]. Although these observational data favoring angiography are subject to selection and survival bias, they suggest that early angiography and PCI may increase the survival rate following cardiac arrest. Selected studies comparing outcomes in patients with and without early coronary angiography are summarized in Table 1.

Trends in Survival Rate After Cardiac Arrest.
IHCA, in-hospital cardiac arrest; OHCA, out-of-hospital cardiac arrest. Data obtained from the American Heart Association statistical update of heart disease and stroke statistics [7].
Selected Studies Comparing the Survival Rate with and without Early Coronary Artery Angiography.
| Authors, year | Region | Design | Sample size | STEMI (%) | Shockable (%) | Early CAG (%) | Timing of CAG | PCI (%) | Survival with CAG (%) | Survival without CAG (%) | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vadeboncoeur et al. [13], 2018 | Arizona, USA, 40 centers | Multicenter registry | 1881 | 28.9 | 52.6 | 43 | Early, <24 h | 13 | 72 | 53 | Propensity-matched OR 2.1 (95% CI 1.30–3.55) for survival with CAG versus without GAG |
| Jaeger et al. [14], 2018 | France | Multicenter registry | 7584 | NA | 24.5 | 39 | Direct to catheterization laboratory | NA | 45.1 | 24 | Survival rate at day 30. Propensity-matched OR 1.52 (95% CI 1.33–1.72) for survival with immediate CAG versus without immediate CAG |
| Bougouin et al. [15], 2018 | Paris, France | Multicenter registry | 1410 | 41 | 50.1 | 72 | Frist few hours | NA | 40 | 13 | Early invasive improved survival in better survival rate in low-risk patients (OR 2.3, 95% CI 1.4–3.9) but not in medium-risk and high-risk patients |
| Shin et al. [16], 2017 | Korea | Multicenter registry | 607 | NA | 70 | 49 | Early, <24 h | NA | 66 | 22 | Propensity-matched OR 2.7 (95% CI 1.36–4.6) for survival rate with early CAG versus without early CAG |
| Kern et al. [11], 2015 | Europe, USA, 34 centers | Multicenter registry | 754 | 26 | 58 | 48 | <2 h | 30 | 56.3 | 34.1 | Survival rate similar between patients with STEMI and without STEMI if they underwent early CAG (54.7 vs 57.9%) Without STEMI: 57.9% |
| Vyas et al. [17], 2015 | USA | Multicenter registry | 4029 | 19.9 | 100 | 48.5 | <1 calendar day | NA | 76 | 59.4 | Propensity-matched OR 1.52 (95% CI 1.28–1.80) for survival with early CAG versus without early CAG |
| Callaway et al. [18], 2014 | USA, Canada | Secondary analysis of RCT | 3981 | 17.5 | 40.7 | 19.2 | <24 h | 17.7 | 64.7 | 27.1 | Multivariate regression analysis: OR 1.69 (95% CI 1.06–1.70) for survival with early CAG versus without early CAG |
| Hollenbeck et al. [19], 2014 | Nashville, USA | Multicenter registry | 269 | 0 | 100 | 45 | <24 h | 21 | 65.6 | 48.6 | Multivariate regression analysis: OR 2.86 (95% CI 1.43–5.6) for survival with early CAG versus without early CAG |
| Søholm et al. [20], 2013 | USA | Multicenter registry | 1020 | 16 | 61 | 27 | <24 h | NA | NA | NA | Univariate OR for 30-day mortality with early CAG versus without early CAG 0.35 (95% CI 0.18–0.70) |
| Strote et al. [21], 2012 | USA, 11 centers | Multicenter cohort | 240 | 34.2 | 100 | 25 | <6 h | 62 | 72 | 49 | Survival rate increased with early CAG versus without early CAG (72 vs. 49%). |
| Bro-Jeppesen et al. [22], 2012 | USA, 1 center | Single-center retrospective study | 269 | 32 | 80 | 44 | <12 h | 21 | 66 | 54 | Early CAG did not increase survival rate in patients without STEMI: OR 0.69 (95% CI 0.4–1.2) |
| Dumas et al. [23], 2010 | Paris, France | Multicenter registry | 435 | 31 | 68 | 46 | Direct to catheterization laboratory | 41 | 51 | 31 | Multivariate regression analysis: successful PCI predicted survival, OR 2.06 (95% CI 1.16–3.66) |
CAG, coronary angiography; CI, confidence interval; NA, not available; OR, odds ratio; PCI, percutaneous coronary intervention; RCT, randomized clinical trial; STEMI, ST elevation myocardial infarction.
Cardiac arrest patients are a diagnostic challenge because of the difficulty in obtaining their history, and limitations in the ability of the post–cardiac arrest electrocardiogram (ECG), echocardiogram, and cardiac biomarkers to identify significant underlying coronary disease [24]. Early coronary angiography facilitates the rapid definitive diagnosis of coronary artery disease and reduces delay to revascularization. Therefore routine angiography may have an important role in the management of cardiac arrest patients.
Out-of-Hospital Cardiac Arrest
ST Elevation Myocardial Infarction
Although randomized trials have demonstrated an increased rate of survival with emergent coronary angiography and PCI in patients with acute STEMI, these studies systematically excluded patients with cardiac arrest [25]. Rather, the evidence in support of early coronary angiography in patients with OHCA comes from observational data. In a report from the PROCAT registry, 96% of patients with ST elevation on the ECG after cardiac arrest had an acute coronary lesion that was amenable to treatment [23]. Numerous observational studies have demonstrated an increased rate of survival to hospital discharge associated with early (same day) angiography in patients with ST elevation after cardiac arrest. Several of these studies also suggested improved neurological outcomes with this strategy. In a pooled analysis of 792 patients with OHCA and ST elevation on ECG, the rate of survival to discharge was 64%, as compared with the historical survival rate of 25–30% when early angiography was not routinely performed [26, 27]. Although the survival rate was significantly lower for comatose patients than for conscious patients, both groups appeared to have significant benefit from early coronary angiography and PCI. In a Slovenian cohort of 135 patients successfully resuscitated from OHCA, Gorjup et al. [28] described a 100% survival rate in conscious patients (36%), all of whom underwent urgent angiography, and a 51% survival rate in comatose patients, 79% of whom underwent urgent angiography. Similarly, in a cohort of 98 patients from the United States with ST elevation after cardiac arrest who underwent emergent angiography, 75% were unconscious but the rate of survival to discharge was 96 vs. 44% and the rate of good neurologic outcome was 96 vs. 39% in conscious versus comatose patients [29]. These studies describe cohorts with a high proportion of witnessed arrests and shockable rhythms, therefore resulting in high survival rates, but suggest that revascularization should be routinely performed in patients with ST elevation even in the comatose patient. As a result of these data, in the 2015 American Heart Association guidelines it is a class I (level of evidence B nonrandomized studies) recommendation that all patients presenting with OHCA and ST elevation on ECG undergo emergent coronary angiography [30].
Most studies have used PCI as the revascularization strategy, but there may be a role for thrombolytics. The only randomized trial addressing the use of thrombolytics in cardiac arrest compared tenecteplase without adjunctive aspirin or heparin with placebo and was stopped prematurely for futility. There was no difference in survival rates, there were similar rates of major nonintracranial hemorrhage (7.7 vs. 6.4%), and there was a higher rate of symptomatic intracranial hemorrhage (0.8 vs. 0%) [31]. Nevertheless, the sum of observational data, comparing thrombolytics with PCI, suggests that despite increased rates of major bleeding, outcomes are similar [32, 33]. Therefore, as with the usual management of STEMI, if significant delay to reperfusion is anticipated, it appears reasonable to administer fibrinolytics unless there is significant concern for bleeding such as in cases of prolonged (>10 min) or traumatic CPR [34].
No STEMI
Up to two-thirds of patients presenting with OHCA do not have ST elevation on ECG, but when they undergo angiography a significant portion are found to have severe coronary artery disease. Spaulding et al. [4] performed early coronary angiography in 84 consecutive survivors of cardiac arrest and found that 60 patients (69%) had culprit lesions, with 40 (48%) of them being recently occluded arteries. One-fourth of the patients with occluded arteries did not have ST elevation or chest pain, suggesting that clinical and ECG criteria alone are not sufficient to rule out acute coronary syndrome as the underlying cause of OHCA. Since this seminal report, the accumulated evidence has led to the American Heart Association and European Society of Cardiology guidelines advocating early coronary angiography and PCI, if indicated, when myocardial ischemia is suspected as the likely cause of cardiac arrest [30, 35].
The benefit of early versus delayed angiography in OHCA patients without STEMI was addressed by a recent meta-analysis of 2133 patients in eight studies, seven of which were observational [36]. Most patients (86%) were survivors of VT/VF arrest, and 940 underwent early angiography (<12 h from presentation). In-hospital mortality was lower in the group receiving early coronary angiography: 19.6 vs. 35.6% (OR 0.46, 95% CI 0.36–0.56). Long-term (6–14-month) mortality was also lower in patients who underwent early coronary angiography: 23.7 vs. 30% (OR 0.59, 95% CI 0.44–74). Furthermore, neurologic outcomes were improved in the early angiography group at hospital discharge (OR 2.00, 95% CI 1.50–2.49) and at 6–14 months (OR 1.48, 95% CI 1.06–1.90). Similarly, in a propensity-matched analysis of the CARES registry including patients with and without STEMI, early angiography was associated with higher odds of survival (OR 1.52, 95% CI 1.28–1.80) and favorable neurologic outcome (OR 1.47, 95% CI 1.25–1.71) [17]. This was confirmed in another propensity score–matched analysis from the Korean CAPTURES registry, which found an OR of 2.3 (95% CI 1.6–3.30) for survival with favorable neurologic outcome [16].
The only published randomized study assessing the benefit of early angiography and PCI in this population is the ARREST pilot randomized trial conducted in the United Kingdom [37]. Forty patients with witnessed VT/VF arrest were randomized to receive early coronary angiography or routine care. Sixty-three percent of patients had significant coronary artery disease, and 47% underwent revascularization. No differences in 30- and 60-day mortality were observed in this study; however, it was likely underpowered to reveal differences.
Several randomized studies to assess the benefit of early coronary angiography in patients with OHCA and no ST elevation on ECG are ongoing. The COACT trial is enrolling 552 patients across 14 centers in the Netherlands, and results are expected later this year [38]. Two smaller multicenter studies are also currently enrolling patients [39, 40].
Presenting Rhythm
The survival rate is higher in patients with VT or VF as their presenting rhythm, as compared with other rhythms (23 vs. 14.8%), but the presenting rhythm is a poor predictor of underlying coronary artery disease [41]. In a meta-analysis of early versus delayed angiography in patients without ST elevation following cardiac arrest, 35% of patients with an initial shockable rhythm underwent PCI compared with 18.6% of patients with an initial nonshockable rhythm, but 47% of patients with a nonshockable rhythm had significant coronary lesions [36]. Likewise, in a series of 203 consecutive patients without ST elevation or new left bundle branch block on ECG after OHCA, obstructive coronary disease was found in 60% of patients in both groups, and the burden of disease by the SYNTAX score was similar between patients with an initial shockable rhythm and patients with a nonshockable rhythm (10.3 vs. 10.2). However, there was a trend toward a higher incidence of acute coronary syndrome (29.7 vs. 16.4%, P=0.054) in the patients with an initial shockable rhythm [42]. Lastly, in a logistic regression analysis from the PROCAT II registry, early PCI was associated with a favorable neurologic outcome, and an initial shockable rhythm was the only variable that independently predicted the requirement for PCI (OR 2.83, 95% CI 1.84–4.36) [43].
In-Hospital Cardiac Arrest
It is reasonable to believe that the cause of IHCA is significantly more heterogeneous than that of OHCA and patients may not derive a similar benefit from early coronary angiography. This subset of patients has a higher proportion of noncardiac causes of cardiac arrest, such as infections, pulmonary embolism, and respiratory failure. In the Get With The Guidelines registry the prevalence of noncardiac comorbidities in patients with IHCA was 45% for respiratory insufficiency, 37% for renal failure, 34% for diabetes, and 18% for sepsis [44]. Compared with the approximately 12% survival rate in OHCA, the rate of survival to discharge after IHCA is higher at approximately 25%, and has significantly increased since 2000, when it was 13.7% (P for trend <0.001). A presenting rhythm of VT/VF was present in 20% of these patients, and was associated with a higher rate of survival to discharge of 33.9% compared with 12.2% when the rhythm was nonshockable [45]. Two single-center studies have examined the use of coronary angiography in IHCA [46, 47]. Merchant et al. [46] performed a retrospective review of 110 patients who were successfully resuscitated after presenting rhythms of VF or pulseless VT. Early angiography was performed in 27% of these patients and was associated with increased rate of survival to discharge: 80 vs. 54% (OR 3.8, 95% CI 1.35–10.90). Helton et al. [47] failed to show a benefit for survival or neurologic outcomes in a review of 116 patients with resuscitated IHCA due to pulseless VT or VF. Ninety-three patients (80%) underwent coronary angiography and 37 underwent PCI, which was associated with a nonsignificant increase in the survival rate (OR 1.54, 95% CI 0.79–3.02). Both studies included only patients with VT/VF; however, the presenting rhythm in more than 75% of IHCAs is pulseless electrical activity, and therefore the benefit of angiography in these patients remains unknown. Although survival rates increased between 2000 and 2011, they appear to have since plateaued (Figure 1). Further research is needed in a larger population of patients to identify the appropriate patient for early coronary angiography and revascularization.
Selection of Patients for Early Coronary Angiography
Most patients with OHCA do not survive, and of those who do, up to 80% remain comatose despite return of spontaneous circulation. More than 98% of conscious survivors of OHCA who underwent early coronary angiography survived to hospital discharge with a good neurologic outcome [9]. Therefore conscious survivors of OHCA do not present a dilemma regarding resuscitation care. When the patient presents comatose, the decision is more difficult and requires a careful consideration of factors that predict poor outcomes to identify patients who may not benefit from early coronary angiography. Because of the critical importance of the time to reperfusion, American Heart Association and European Society of Cardiology guidelines recommend that all cardiac arrest survivors with STEMI should undergo emergent coronary angiography even if they are comatose, without commenting on the features of the presentation or the cardiac arrest [30, 48]. The Interventional Council of the American College of Cardiology is more measured in its algorithm for management of the comatose patient, recommending that suitability for benefit be assessed before angiography is performed [49]. When there is no ST elevation on ECG, there is broad agreement between societal guidelines that patients should undergo evaluation to assess them for noncardiac causes and unfavorable features of resuscitation that would predict futility. Several predictors of outcomes (features of the cardiac arrest, biochemical markers, and patient-specific factors) are reviewed in detail in the following paragraphs and a suggested approach for making the decision to perform urgent coronary angiography is outlined in Figure 2.
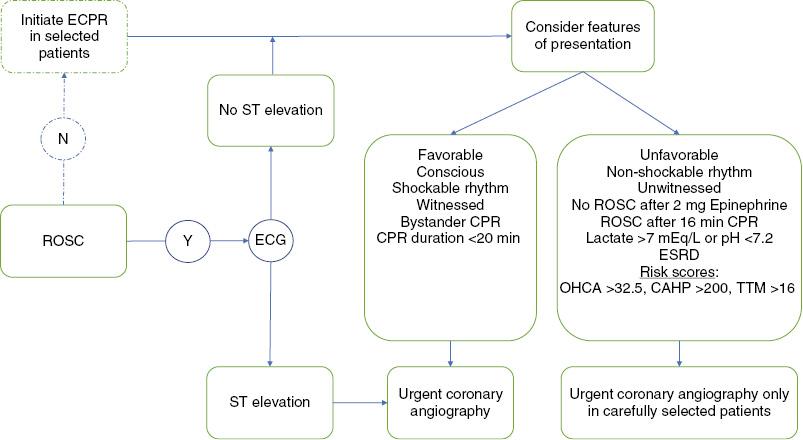
Selection of Patients for Coronary Angiography After Out-of-Hospital Cardiac Arrest (OHCA).
CAHP, cardiac arrest hospital prognosis risk score; CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; ECPR, extracorporeal cardiopulmonary resuscitation; ESRD, end-stage renal disease; ROSC, return of spontaneous circulation; TTM, Target Temperature Management risk score.
Jabre et al. [50] analyzed two registry groups and one randomized study and identified three features of the cardiac arrest that predicted death following OHCA with nearly 100% specificity: (1) cardiac arrest not witnessed by EMS personnel, (2) nonshockable initial cardiac rhythm, and (3) no return of spontaneous circulation before the third round of epinephrine administration. Only one patient meeting all three criteria survived. When the criteria were validated in two external cohorts totaling 1536 patients, there were no survivors among the patients in whom all three characteristics were present.
Approximately 50% of cardiac arrests are witnessed. In the CARES registry of OHCA, the survival rate of patients with unwitnessed cardiac arrest was less than 5% and was independent of the EMS response time [51]. In a meta-analysis of studies reporting outcomes of OHCA, the survival rate in unwitnessed cardiac arrest was 6.4% compared with 13.5% in witnessed cases [41]. Although bystander CPR is associated with an increased survival rate (OR 1.23, 95% CI 1.14–1.33) only half of the patients with a witnessed cardiac arrest received it [52]. When the presenting rhythm is shockable, the survival rate is up to 67% as compared with 22% when the rhythm is nonshockable [53]. The time to return of spontaneous circulation is another strong predictor of survival, as the probability of good functional outcome falls to 1% after 16 min of CPR [54].
Elevated serum lactate and low pH levels reflect cellular hypoxia due to prolonged hypoperfusion, and these biomarkers have been established as predictors of poor prognosis. A lactate level greater than 7 mEq/L and pH below 7.2 have been suggested as thresholds identifying patients less likely to benefit from coronary angiography and PCI [49]. In the PROCAT registry of OHCA, one-third of patients had a lactate level greater than 7 mEq/L, and this was associated with a significantly lower rate of survival to hospital discharge (OR for each quartile increase in lactate level 0.55, 95% CI 0.44–0.70) [23]. Similar findings were observed in a retrospective study of patients with IHCA, in which survivors with good neurologic outcome had lower mean initial (1.9 mEq/L vs. 5.3 mEq/L) and peak (3.9 mEq/L vs. 8.8 mEq/L) lactate levels as compared with patients with poor neurologic outcome [55]. A lactate level greater than 7 mEq/L results in a pH below 7.2, and therefore severe acidosis is predictive of poor neurologic outcomes as well. In a group of 196 OCHA and ICHA survivors, a pH below 7.2 predicted a three-fold increase in poor neurologic outcome (OR 3.3, 95% CI 1.28–8.45) in patients with an initial shockable rhythm following cardiac arrest [56].
There are patient-specific factors that predict particularly poor outcomes and warrant further consideration of risks and benefits before coronary angiography. Patients with end-stage renal disease have an extremely poor prognosis after cardiac arrest. Among 110 cardiac arrests that occurred at dialysis facilities in the Seattle/King County area, 84% of which were witnessed, only 24% of patients survived to hospital discharge and only 15% survived to 1 year [57]. Age is a prognostic indicator as well, and the treatment algorithm from the Interventional Council of the American College of Cardiology suggests that age greater than 85 years should be considered as unfavorable, but the data do not support a distinct cut point. This threshold is derived from a study of the National Registry of Cardiopulmonary Resuscitation that included 49,130 cases of IHCA [58]. A U-shaped relationship was observed, with mortality higher in the 18–39-year-old and 80 years and older groups compared with the 40–79-year-old group, with no clear age cutoff. In the INTCAR registry of OHCA and IHCA, 29% of survivors older than 75 years were discharged with good neurologic outcome compared with 42% of survivors aged 18 to 75 years; however, the difference was not significant when the presence of do not resuscitate orders was considered. Among survivors, there were no differences in the rates of good neurological outcome in the two age groups [59]. A large Danish study reported that OHCA patients older than 80 years had a lower survival rate than younger patients; however, older patients underwent coronary angiography less frequently, and the rate of survival to discharge with favorable neurologic outcome was similar to that of younger patents [60]. These findings suggest that reports of poorer outcomes in the elderly may be partially related to differences in treatment, and physiologic features and comorbidities may be more important than age in determining prognosis.
Any single feature of unfavorable resuscitation may not be predictive in an individual patient, and a combination of features may be more robust; therefore several risk scores have been developed to predict patient prognosis following cardiac arrest. A favorable outcome is defined in these studies as survival with a cerebral performance category of 1 (conscious and normal) or 2 (conscious with moderate disability) and poor outcome is defined as a cerebral performance category greater than 2 (i.e., death, coma, or survival with severe disability). The OHCA score was developed in a cohort of 130 consecutive patients admitted to a single French intensive care unit and is computed as the sum of five parameters and ranges from −30 to 60, with a score greater than 32.5 having a positive predictive value (PPV) of 90% in identifying patients with a poor neurologic outcome. The area under the receiver operating characteristic curve was 0.88 in a validation cohort, and has since been externally validated, demonstrating good performance, with an area under the curve of 0.77 [61, 62]. The cardiac arrest hospital prognosis (CAHP) score was developed in a larger cohort of 859 patients from Paris, France, and performed well in identifying those with a poor outcome [63]. A CAHP score cutoff of 200 points had a PPV of 98%. The Target Temperature Management score leveraged data from 933 patients in the Target Temperature Management trial and uses 10 variables to calculate a score ranging from −2 to 35. A score of more than 16 had a PPV of 91% for a poor neurologic outcome, with a specificity of 95–96% [64]. The cardiac arrest survival postresuscitation in-hospital (CASPRI) risk score was developed to predict the likelihood of survival among patients with IHCA [44]. This score ranges from 0 to 50, and 11 characteristics of the cardiac arrest and resuscitation are used to calculate it. A score greater than 27 predicted a probability of neurologically favorable survival of only 2.8%. All of these risk models have limitations, including the use of measures such as the time to CPR, the time of CPR, and the time to return of circulation, which are notoriously unreliable. However, they may be useful tools to inform discussions with the patient’s family regarding prognosis and to guide treatment options such as coronary angiography.
Extracorporeal Cardiopulmonary Resuscitation
In patients who do not achieve return of spontaneous circulation because of refractory VT/VF but have otherwise favorable prognostic factors, there may be a role for coronary angiography, but it is challenging to perform during ongoing CPR. There is growing interest in the initiation of extracorporeal CPR using percutaneously placed devices that provide mechanical circulatory support and extracorporeal membrane oxygenation. This allows hemodynamic stabilization and end-organ perfusion that allows time for coronary angiography and PCI. In a series of 55 patients presenting to the catheterization laboratory after an average of 58 min of CPR, extracorporeal life support was initiated in 91% of patients and appeared to increase the rate of survival with good neurologic outcome from 15.3 in historical controls to 42% [65]. An acute thrombotic lesion was identified in 64% of these patients, and PCI was performed in 84%. A systematic review of observational studies of extracorporeal CPR initiated in patients with refractory cardiac arrest reported an overall survival rate of 22%, with 13% having good neurologic recovery [66]. Caution is warranted in interpreting these results since there was significant heterogeneity in the patient population, protocols, and outcomes measured between the studies.
Conclusion
Survival rates after cardiac arrest have not increased in the past decade. Coronary artery disease is commonly implicated as the underlying cause in cardiac arrest, particularly when patients present with ventricular tachyarrhythmia or ST elevation on ECG; therefore early revascularization may improve outcomes. Acute coronary lesions are found in approximately half of patients undergoing angiography, and there are mounting, mostly observational, data that PCI increases the rate of survival to discharge and improves neurologic outcomes even if the patient is comatose after return of spontaneous circulation. In patients with ST elevation on ECG, immediate angiography is recommended. When there is no ST elevation, selective early angiography should be considered after evaluation of the prognostic features of the patient and the cardiac arrest.
Key Points
Sudden cardiac death is most commonly due to ventricular arrhythmias in the presence of coronary artery disease.
The rate of survival after OHCA is approximately 12% and has not significantly increased over time. In comparison, the rate of survival after IHCA increased from 13.7 in 2000 to 25% in 2011, where it has plateaued.
Acute coronary (culprit) lesions are found in most patients undergoing early coronary angiography after cardiac arrest, and are more likely to be present when there is ST elevation on EGG or when the presenting rhythm is shockable (i.e., VT or VF).
When the post–cardiac arrest EGG shows ST elevation, patients are recommended to undergo coronary angiography and PCI if indicated, similar to the recommendation for patients without cardiac arrest.
Early coronary angiography and PCI within 12 h of presentation has been associated with an increased rate of survival in patients with cardiac arrest without ST elevation on EGG even if they are comatose on admission.
Clinical variables and prognostic models predict patient outcomes following cardiac arrest. In patients without ST elevation on EGG, an evaluation of the risks and benefits is recommended to identify patients likely or unlikely to benefit from angiography and PCI.
