Introduction
Since the first cardiac pacemaker was implanted in the human body in 1958, pacemaker technology has been continuously improved to be a mainstay for the treatment of many major clinical problems, such as sick sinus syndrome and high-degree atrioventricular block. However, the pocket- and lead-related complications resulting from traditional pacemakers, such as infection, hematoma, incision dehiscence, and pocket effusion, have gradually gained attention [1].
To reduce the occurrence of these complications, the concept of leadless pacemakers was proposed in the 1970s [2]. With further development, the leadless pacemakers currently in use include two types: (1) the Nanostim™ leadless cardiac pacemaker (LCP) [3] and (2) the Micra™ transcatheter pacing system (TPS) [4]. The Nanostim LCP is manufactured by St. Jude Medical and integrates pacemaker devices, lithium batteries, and electrodes. The length of the Nanostim LCP is 42 mm, the maximum diameter is 5.99 mm, and the weight is 2 g. The Nanostim LCP is delivered from the femoral vein to the right ventricle through an 18 F (inner diameter)/21 F (outer diameter) catheter. The Nanostim LCP uses conductive communication to minimize battery consumption, and its battery life is 8.5–9.8 years [3]. The Micra TPS, manufactured by Medtronic, has a length of 25.9 mm, an external diameter of 6.7 mm, and a weight of 2 g. The guide sheath of the Micra TPS is a 23 F (inner diameter)/27 F (outer diameter) catheter. The Micra TPS uses traditional radiofrequency current methods, and its battery life is 4.7–9.6 years [4]. In terms of equipment extraction, a leadless pacemaker has a special controllable catheter for extraction [3, 4]. With the increasing use of leadless pacemakers, Mengi [5] reported that leadless pacemakers have a low risk of major complications and Cantillon et al. [6] discovered an alarmingly high incidence of cardiac perforation or pericardial effusion and vascular events on short-term follow-up.
It is not clear which of the two pacemaker systems is better. Therefore, this meta-analysis was conducted to answer this question.
Methods
Search Strategy
A systematic search in PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the CNKI database, and the Wanfang database was performed from July 2013 to December 2019. Studies eligible for inclusion were identified by the following search strategy: first run, “leadless pacemaker” OR “leadless cardiac pacemaker” OR “Micra transcatheter pacing” OR “leadless pacing” OR “leadless cardiac pacing”; second run, “traditional pacemaker” OR “conventional pacemaker” OR “permanent pacemaker” OR “standard pacemaker”; third run, “effect” OR “therapeutic effect” OR “treatment outcome” OR “treatment effect” OR “therapeutic efficacy” OR “efficacy” OR “complication”; fourth run, combination of the search terms for the first, second, and third runs.
Selection Criteria
The method used in this meta-analysis is in accordance with the guidelines of the Cochrane Collaboration [7].
Inclusion Criteria and Exclusion Criteria
The exclusion criteria were as follows: duplicate literature; single-arm study; raw research data cannot be obtained or studied; review, case report, or animal experiments; languages other than English or Chinese.
The inclusion criteria were as follows: the studies must be designed as a head-to-head comparison of traditional pacemakers with leadless pacemakers; detailed data can be extracted to compare the primary and secondary end points; English or Chinese language.
End Points
The primary end point was major complications, which were defined as system- and procedure-related events resulting in death, permanent loss of device function, hospitalization, hospitalization prolonged by 48 hours, or system revision (excluding pocket- and lead-related complications).
The secondary end points were cardiac perforation/pericardial effusion, device revision or extraction, loss of device function, and death.
Literature Screening, Data Extraction, and Quality Evaluation
Diyu Cui and Yunqing Chen independently performed the literature screening, data extraction, and methodological quality evaluation. A consensus was reached through discussion or with the assistance of a third party. A self-made data extraction table was used to extract the data. The extracted content included mainly (1) the general characteristics and basic conditions of the research, (2) The study source of enrolled researches, (3) the specific method used for the intervention, and (4) the clinical outcome index. Finally, the Newcastle-Ottawa Scale [8] was used to evaluate the risk and the quality of the studies.
Statistical Analysis
Data processing was performed with Rev Man 5.3 from the Cochrane Collaboration. The count data were analyzed by the risk ratio (RR) and the 95% confidence interval (CI) as the effect size. Heterogeneity was determined by the χ 2 test. When P≥0.05 and I 2≤50%, the random effects model was used for meta-analysis; when P<0.05 and I 2>50%, the cause of heterogeneity was first searched for, which may have led to subgroup analysis of heterogeneity factors. The clinical heterogeneity was evaluated by the study background, the basic characteristics of the study population, the type of implanted pacemaker, and so on. If there was statistical heterogeneity between the study results, then a random effects model meta-analysis was used, and the results were interpreted with caution. Descriptive analysis was performed if the data could not be combined.
Results
Screening of Studies
In our database research, 178 citations were retrieved: 53 articles were from PubMed, 73 articles were from Embase, three articles were from the CNKI database, ten articles were from CENTRAL, and 39 articles were from the Wangfang database. Thirty-two references were excluded because of references duplicated by EndNote. One hundred thirty-six clearly irrelevant references were excluded through reading of the titles and abstracts. Therefore, ten references remained for further review. After reading of the full text, four references were excluded: three references had a repeated population, and for one reference the full text could not be accessed. Finally, six studies [6, 9–13] were included in the systematic review (Figure 1, Table 1).
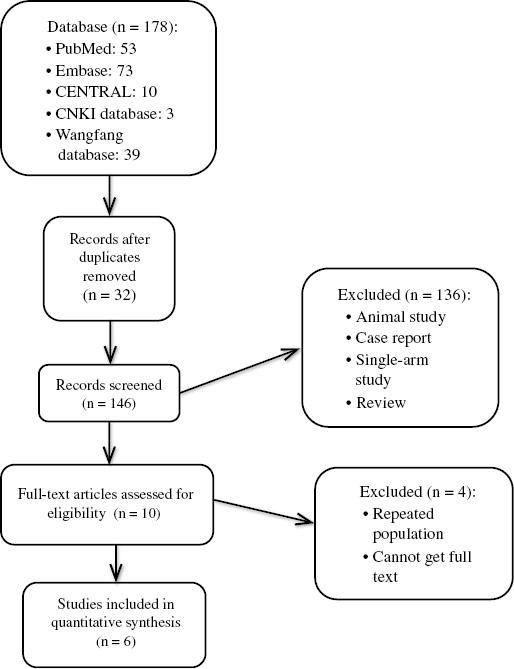
Selection of Studies for Inclusion in the Meta-Analysis.
CENTRAL, Cochrane Central Register of Controlled Trials.
Baseline Characteristics of the Studies.
| Study | Year | Study design | Study source | Participants | Age (years) | Male sex (%) | Follow-up (days) | NOS score | End point | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LP | TP | LP | TP | LP | TP | LP | TP | LP | TP | LP | TP | ||||
| Cantillon et al. [6] | 2018 | Prospective, nonrandomized, multicenter clinical trial | NA | LEADLESS II IDE study | Truven Health MarketScan Research database | 718 | 1436 | 75.6±11.9 | 76.1±12.3 | 62 | 63 | 30 | NA | 7 | ② |
| Kamath et al. [9] | 2018 | Retrospective study | – | 32 | 30 | 70 | 81 | 59 | 31 | 458 | 6 | ②+③ | |||
| El-Chami et al. [10] | 2018 | Prospective, nonrandomized, multicenter study | NA | Micra Post-approval Registry | Medtronic trials | 1817 | 2667 | 75.6±13.5 | NA | 61 | NA | 204±207 | NA | 5 | ①+③+④+⑤ |
| Carabelli et al. [11] | 2018 | Single-center controlled study | – | 72 | 72 | NA | NA | NA | NA | 180 | 180 | 6 | ① | ||
| Vaidya et al. [12] | 2019 | Prospective study* | NA | * | Mayo Clinic Rochester | 90 | 90 | 80.5 (74–86)† | 78.2 (73.8–85.3)† | 37 | 37 | 62 (28–169)† | 6 | ①+②+④+⑤ | |
| Gonzalez-Melchor et al. [13] | 2019 | Single-center, prospective, observational study | – | 133 | 178 | NA | NA | 60 | NA | 7 | ①+③ | ||||
①, major complication; ②, cardiac perforation/effusion; ③, death; ④, device revision/extraction; ⑤, loss of device function; LP, leadless pacemaker; NA, not applicable; NOS, Newcastle-Ottawa Scale; TP, traditional pacemaker.
*MICA lead-less pacemaker IDE; Nanostim study.
†Median (interquartile range).
Characteristics of Included Studies
The basic features of the included studies are shown in Table 1. Six studies were included, and none of these were a randomized controlled trial. In three studies, the single-arm test population for leadless pacemakers was selected as the experimental group and the single-arm test population for traditional pacemakers was selected as the control group to conduct a comparative study in a paired way. The study by Carabelli et al. [11] was a single-center controlled study, the study by Gonzalez-Melchor et al. [13] was as a single-center, prospective, observational study, and the study by Kamath et al. [9] was a retrospective cohort study.
Data Extraction
Primary End Point (Major Complications)
Among the six included studies, four conducted quantitative analysis of major complications. For major complications, the definitions were not consistent across the studies. The main complications in the study by Vaidya et al. [12] were severe adverse events such as death, cardiac arrest, stroke, pericardial effusion requiring intervention, hematoma, or vascular tear requiring surgical intervention. The major complications in the study by El-Chami et al. [10] were system- and procedure-related events that resulted in death, permanent loss of device function, hospitalization, extended stay of 48 hours, or system revision. The other two major complications were defined as serious adverse events resulting from system- and procedure-related events. Therefore, the main complications in this study were defined as serious adverse events related to systems and procedures, such as death and cardiac arrest.
Major Complications with Leadless Pacemakers versus Traditional Pacemakers
Among the six studies for quantitative analysis, we were able to extract complete data on major complications from four studies. The results of the meta-analysis suggested that leadless pacemakers have a lower incidence of major complications than traditional pacemakers (RR 0.33, 95% CI 0.25–0.44, P<0.00001, I 2=49%) (Figure 2).

Major Complications.
CI, confidence interval; df, degrees of freedom; LP, leadless pacemaker; M-H, Mantel-Haenszel; TP, traditional pacemaker.
Secondary End Points
We pooled the effect estimates of cardiac perforation/pericardial effusion from three studies, which suggested that a leadless pacemaker is associated with a higher risk of cardiac perforation/pericardial effusion (RR 4.28, 95% CI 1.66–11.08, P=0.003, I 2=0%) (Figure 3).
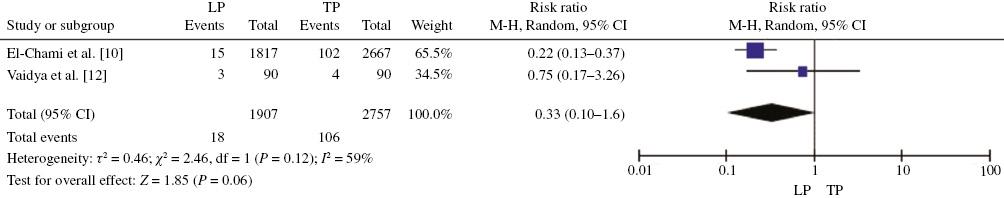
Finally, the meta-analysis showed no statistically significant difference for death between leadless pacemakers and traditional pacemakers (RR 1.59, 95% CI 0.46–5.54, P=0.46, I 2=78%) (Figure 4). For the two research indicators of device revision or extraction and loss of device function, the combined analysis results indicated that there was no statistically significant difference between leadless pacemakers and traditional pacemakers in these two concurrent aspects (device revision or extraction, RR 0.33, 95% CI 0.10–1.06, P=0.06, I 2=59%; loss of device function, RR 5.19, 95% CI 0.17–161.77, P=0.35, I 2=67%) (Figures 5 and 6).

Death.
CI, confidence interval; df, degrees of freedom; LP, leadless pacemaker; M-H, Mantel-Haenszel; TP, traditional pacemaker.
Publication Bias and Sensitivity Analysis
In accordance with the Cochrane handbook [14], we did not perform publication bias analysis because of the low number of included studies. We performed sensitivity analysis, which indicated that the results for major complications and cardiac perforation or pericardial effusion were not stable. During sensitivity analysis, it was found that all studies except that of El-Chami et al. [10] had a large impact on the results for major complications, and when the study by Cantillon et al. [6] was excluded, there was a large change in the cardiac perforation or pericardial effusion group, mainly because of the large sample size of these two studies.
Discussion
The leadless pacemaker was developed to avoid the pocket- and lead-related complications caused by traditional pacemakers. Research on the Nanostim LCP and Micra TPS is gradually increasing. Although most of the single-arm study results suggested that the leadless pacemaker has better safety, comparisons of traditional pacemakers and leadless pacemakers are still lacking, especially since randomized controlled trials have not yet been published. This article is the first meta-analysis to compare the differences in clinical complications between leadless pacemakers and traditional pacemakers. The final results suggest that leadless pacemakers are associated with a lower incidence of major complications than traditional pacemakers. However, in terms of cardiac perforation or pericardial effusion, leadless pacemakers have a higher risk. In terms of device revision or extraction, loss of device function, and death, since there were few current studies, no statistically significant differences were found.
In terms of major complications, although the six studies included have different definitions of major complications, they all refer to serious complications caused by equipment or procedures as a whole. Our meta-analysis indicated that leadless pacemakers are associated with a lower incidence of major complications. Given that the follow-up duration of the included studies was about 12 months, leadless pacemakers may be superior to traditional pacemakers with regard to short-term benefits.
The meta-analysis of cardiac perforation or pericardial effusions suggested that a leadless pacemaker was more likely to cause them. The implantation processes for the Nanostim LCP and the Micra TPS are similar, using a catheter-based percutaneous femoral artery approach to introduce the leadless pacemaker and deliver it to the right ventricle. After the right ventricle has been reached, the leadless pacemaker is fixed to the ventricular wall by different means: the Micra TPS [15] has a tine-based fixation mechanism, and the Nanostim LCP is fixed with a helical screw. This fixation method has a certain risk: if the fixed position is too deep or too shallow, it is prone to result in heart perforation or device displacement, which is inseparable from the proficiency of the operator. The relatively high perforation rate was thought to be associated with deploying a catheter or fixed tooth through the right ventricular free wall. Therefore, implantation training should be focused on ventricular septal deployment [16]. A study on the Nanostim LCP demonstrated that learning curves exist for Nanostim LCP implantation. Procedure efficiency increased with increased operator experience, according to a decrease in the incidence of serious adverse device effects, procedure duration, and number of repositioning attempts [17]. This shows the impact of proper training and gaining experience on the performance learning curve for the Nanostim LCP.
For death, only three articles could be included in the meta-analysis, and the final results are not statistically different. Death is defined mainly as death due to procedures or equipment. Large-sample control experiments for death are still lacking. Only one of the three samples was relatively large, so it may cause greater heterogeneity.
We found no statistically significant differences with regard to device revision or extraction and loss of device function complications because of the lack of current control studies. In current research, the occurrence of device battery failure has attracted much attention, especially for the Nanostim LCP. Lakkireddy et al. [18] reported that there were 34 battery failures in 1423 Nanostim LCP implants, seven of which directly led to the global emergency recall and termination of the Nanostim LCP. The higher-than-expected battery failure can directly threaten the implant’s life and safety [19]. In the case of equipment failure and the need for replacement, the safety of leadless pacemaker extraction has also been studied. In the review by Li et al. [20], according to the available data, a leadless pacemaker can be extracted at least 4 or 5 years after implantation, but with the development of the leadless pacemaker, device extraction may become more and more convenient.
In this study, by combining the results of currently available comparative studies to analyze the differences in complications between traditional pacemakers and leadless pacemakers, we found that leadless pacemakers have an advantage in terms of lead- and pocket-related complications. In terms of other complications, there is still controversial differences between leadless pacemakers and traditional pacemakers. At the same time, because of the lack of long-term follow-up data for leadless pacemakers, safety is currently unknown in terms of long-term complications. Therefore, there is a great need for more head-to-head studies or randomized controlled trials to guide clinical practice.
Limitations
There were no randomized controlled studies in the included studies, the follow-up duration of the studies was different, and all studies had short-term and medium-term complications. There are no data on studies of leadless pacemakers in China. For the Asian population, 36 people in the Japanese population and 690 people in the rest of the population have been followed up for 1 year, with major complications suggested. Limited by the number of related studies and available data, our major complications were defined not by the incidence but by a comprehensive consideration of the severity and prevalence on the basis of the included studies. In consideration of the standard criteria of major complications in each study, we think that the impact of the difference on the RR is small, and the pooled effect by meta-analysis has certain clinical significance in safety assessment. There is no significant difference in the probability, and the rest of the results will be explored by more relevant clinical trials.
Conclusion
Our meta-analysis appears to favor leadless pacemakers over traditional pacemakers with regard to major complications. This indicates that leadless pacemakers have potential for future clinical applications. However, the application of a leadless pacemaker is still controversial, and more randomized controlled studies are warranted to explore safety and practicality.