Introduction
In December 2019, an outbreak of COVID-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan (Hubei, China) and spread rapidly [1]. There have since been COVID-19 outbreaks in many other countries in the world. On March 11, 2020, the WHO declared COVID-19 a global pandemic. Without efficient medicine, early detection and isolation is essential for SARS-CoV-2 infection control. However, as more secondary and tertiary cases have appeared, the contact history is usually not clear. Even clinical manifestations may be atypical under certain circumstances. Besides, because of an overwhelmed health system, reverse transcription–polymerase chain reaction (RT-PCR) for the detection of SARS-CoV-2 nucleic acid cannot provide quickly screening. In such a situation, chest computed tomography (CT) has been highly recommended for screening of patients with suspected SARS-CoV-2 infection in China [2].
Heart failure is one of the most common diseases encountered by emergency physicians and cardiologists in emergency departments or clinics. Some COVID-19 patients develop heart failure, and the risk of COVID-19 may be higher in heart failure patients [3]. In the current COVID-19 epidemic, frontline physicians will encounter patients with heart failure or COVID-19 pneumonia with atypical clinical features. So it will be a great challenge for physicians to distinguish COVID-19 pneumonia from heart failure at the initial contact. Pulmonary edema caused by heart failure can appear as exudative disease on CT scanning, and is sometimes difficult to distinguish from other exudative disease in clinical practice. Here, with the aim to improve the diagnosis of COVID-19 pneumonia at the initial medical contact, we present the clinical and imaging features of patients with heart failure and patients with COVID-19 pneumonia and one patient with both diseases to clarify the characteristics of heart failure and COVID-19 pneumonia.
Methods
Study Design and Participants
This is a retrospective clinical study. We included consecutive patients with a diagnosis of COVID-19 pneumonia or heart failure who had undergone CT at the initial medical contact at the Second Xiangya Hospital of Central South University from December 1 to March 31, 2020. Patients with negative CT findings and younger than 18 years were excluded. The clinical history, laboratory characteristics, and epidemic characteristics were collected for 23 patients with heart failure and 23 patients with COVID-19 pneumonia. Besides, one patient with both heart failure and COVID-19 pneumonia was also enrolled. All the COVID-19 patients received their diagnosis in fever clinics and were then transferred to the Second Xiangya Hospital of Central South University.
The diagnosis and classification of heart failure were based on the 2016 European Society of Cardiology guidelines [4], and the diagnosis of COVID-19 pneumonia was confirmed by the detection of SARS-CoV-2 in respiratory specimens with at least three positive RT-PCR results. Impaired heart function was defined as heart failure of class II or greater in the Killip or New York Heart Association classification.
Clinical records, laboratory findings, and chest CT scans for all patients were obtained at the initial medical contact. Two study investigators independently checked all the data. Throat-swab specimens from the upper respiratory tract were obtained and tested for SARS-CoV-2 with the kit (BioGerm, Shanghai, China) recommended by the Chinese Center for Disease Control and Prevention following WHO guidelines for RT-PCR. RT-PCR was repeated at least two times with different specimens.
This study was reviewed and approved by the Medical Ethical Committee of the Second Xiangya Hospital of Central South University (approval number 2020005), which waived the requirement for patients’ informed consent in accordance with the guidelines of the Council for International Organizations of Medical Societies.
Image Interpretation
Two thoracic radiologists blinded to the clinical data reviewed the CT images independently and resolved discrepancies by consensus. All images were viewed on both lung (width, 1500 HU; level, −700 HU) and mediastinal (width, 350 HU; level, 40 HU) settings. The presence or absence of imaging features was recorded: ground-glass opacity (GGO), consolidation, rounded morphology, crazy paving pattern, peribronchovascular thickening, septal thickening, fissural thickening, small pulmonary vein enlargement, cardiac enlargement, lesion distribution, lobe involvement, and bilateral lung disease. The detailed definitions of the aforementioned features were as described previously [5, 6].
Statistical Analysis
Statistical analysis was done with SPSS Statistics, version 25.0. Continuous variables were directly expressed as the mean ± standard deviation. Categorical variables were expressed as the number and percentage. Means for continuous variables were compared by independent group t tests when the data were normally distributed; otherwise, the Mann-Whitney test was used. Proportions for categorical variables were compared with the χ 2 test or Fisher’s exact test.
Results
Clinical Information
The study included 23 patients with heart failure and 23 patients with COVID-19 pneumonia. The mean age of the patients with heart failure was 65 years, and 15 (65%) were male, while the mean age of the patients with COVID-19 was 56 years, and 14 (61%) were male. As shown in Table 1, there was no difference in age or sex between the two groups. Every patient in the COVID-19 group had a contact history, while only two patients in the heart failure group had a contact history. More patients had fever or respiratory symptoms in the COVID-19 group than in the heart failure group (100% vs. 39%, P=0.005). In addition, more patients had cardiovascular disease in the heart failure group than in the COVID-19 group (100% vs. 17%, P=0.00065). All the COVID-19 patients had at least three positive RT-PCR results for SARS-CoV-2, while the RT-PCR results were negative for all the patients with heart failure.
Basic Clinical Information in Patients with Heart Failure and COVID-19.
| Heart failure | COVID-19 | P | |
|---|---|---|---|
| Age (years) | 65±14 | 56±17 | 0.110 |
| Male sex | 15 (65%) | 14 (61%) | 0.760 |
| Epidemic contact | 2 (9%) | 23 (100%) | 0.00069 |
| Fever or respiratory symptoms | 9 (39%) | 23 (100%) | 0.005 |
| Cardiovascular disease | 23 (100%) | 4 (17%) | 0.00065 |
| Impaired heart function | 23 (100%) | 3 (13%) | 0.00086 |
| Class II | 16 (70%) | 3 (13%) | |
| Class III | 5 (22%) | 0 (0%) | |
| Class IV | 2 (7%) | 0 (0%) | |
| SARS-CoV-2 positive | 0 (0%) | 23 (100%) | 0.00077 |
Values are the mean ± standard deviation or the number and the percentage of patients.
SARS-CoV-2, severe acute respiratory syndrome coronavirus disease 2.
Imaging Features
Figure 1 shows the typical imaging for heart failure (Figure 1A and 1B) and COVID-19 pneumonia (Figure 1C and 1D). There were some similarities and differences between these two diseases. The imaging features are compared in Table 2. Twenty-two of the patients in the heart failure group had GGO as did 21 of the patients in the COVID-19 group, and there was no statistical difference between the two groups (96% vs. 91%, P=0.550). There were also no differences between the groups with regard to consolidation, crazy paving pattern, and septal thickening. Hoverer, fewer patients had a rounded morphology (4% vs. 70%, P=0.00092) (Figure 1C and 1D) and more patients had peribronchovascular thickening (70% vs. 35%, P=0.018) (Figure 2A and 2B) and fissural thickening (43% vs. 4%, P=0.002) (Figures 1B and 2A) in the heart failure group than in the COVID-19 group. For lesion distribution, although there was no difference in the central distribution between the groups, patients with heart failure were less likely to have a peripheral distribution (30% vs. 87%, P=0.00085). Importantly, 14 patients in the heart failure group had a gravity-dependent gradient distribution (Figure 1A), while only one patient in the COVID-19 group had such a distribution (P=0.00063). For the lobes affected, there was no statistical difference in the frequency of the involvement of two or more lobes or bilateral lung disease. Importantly, there were more patients with pulmonary vein enlargement in the heart failure group than in the COVID-19 group (61% vs. 4%, P=0.00087) (Figure 2C), and there were more patients with subpleural effusion and cardiac enlargement in the heart failure group than in the COVID-19 group (50% vs. 0% and 61% vs. 4%, respectively). Besides, more fibrous lesions were found in the COVID-19 group although there was no statistical difference (22% vs. 4%, P=0.08) (Figure 2E). Figure 2 shows different imaging features for heart failure (Figure 2A–2C) and COVID-19 (Figure 2D–2F).
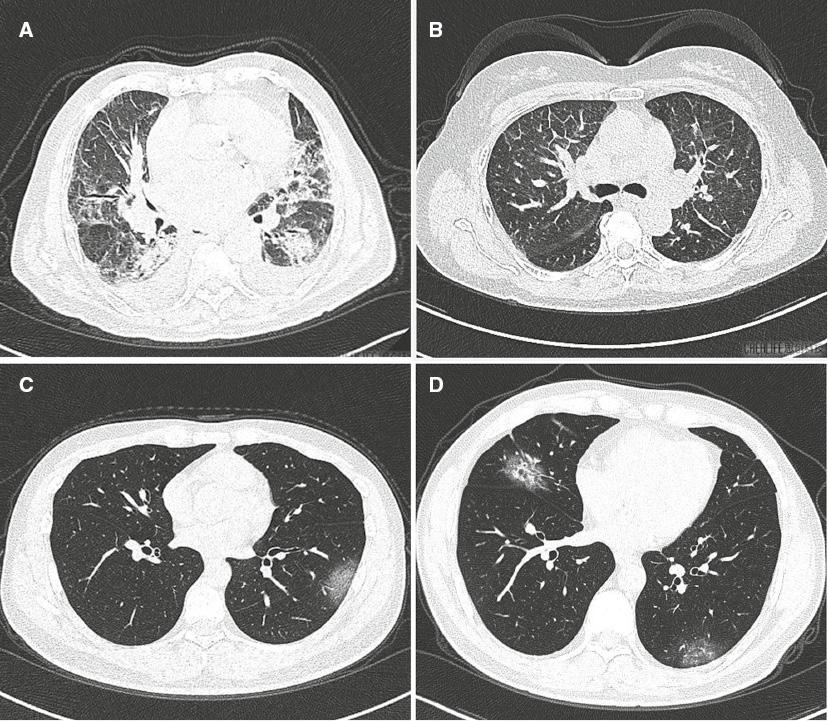
Typical Imaging for Heart Failure and COVID-19 Pneumonia.
(A) Heart failure with bilateral diffuse disease. The disease presented with a gradient distribution and partial ground-glass opacity (GGO) and consolidation inside, accompanied by subpleural effusion. Peribronchovascular thickening and septal thickening were also found. (B) Heart failure with bilateral scattered GGO disease. Peribronchovascular thickening, clear interlobular septal thickening, and fissural thickening were found without subpleural effusion. (C) COVID-19 with single rounded subpleural GGO in the left lung. (D) COVID-19 with rounded GGO in both lungs; partial consolidation was found inside.

Different Imaging Features for Heart Failure (A–C) and COVID-19 (D–F).
(A) Big patchy ground-glass opacity (GGO) in the left lung and sporadic GGO in the right lung. Peribronchovascular thickening and fissural thickening (arrow) were also found, accompanied by subpleural effusion. (B) Subpleural GGO with band-shaped morphology (arrow) in the dorsal segment of both lungs. Interlobular septal thickening and cardiac enlargement were also found. (C) Patchy lesion with a crazy paving pattern in the upper lobe of the right lung with partial consolidation inside. Small pulmonary vein enlargement (arrow) was also found, accompanied by subpleural effusion in the right lung. (D) Big patchy GGO in the left lung. Interlobular septal thickening was also found inside. (E) Multiple disease mixed with GGO and consolidation in both lungs. A fibrous lesion (arrow) was found in the left lung. (F) Patchy lesion with a crazy paving pattern in the upper lobe of the left lung with partial consolidation inside.
Comparison of Imaging Features of Heart Failure and COVID-19 Pneumonia.
| Heart failure | COVID-19 | P | |
|---|---|---|---|
| Ground-glass opacity | 22 (96%) | 21 (91%) | 0.550 |
| Rounded morphology | 1 (4%) | 16 (70%) | 0.00092 |
| Fibrous lesions | 1 (4%) | 5 (22%) | 0.080 |
| Consolidation | 8 (35%) | 14 (61%) | 0.077 |
| Crazy paving pattern | 7 (30%) | 6 (26%) | 0.743 |
| Pulmonary vein enlargement | 14 (61%) | 1 (4%) | 0.00075 |
| Peripheral distribution | 7 (30%) | 20 (87%) | 0.00085 |
| Gradient distribution | 14 (61%) | 1 (4%) | 0.00063 |
| Central distribution | 11 (48%) | 2 (9%) | 0.003 |
| Septal thickening | 22 (96%) | 20 (87%) | 0.295 |
| Fissural thickening | 10 (43%) | 1 (4%) | 0.002 |
| Peribronchovascular thickening | 16 (70%) | 8 (35%) | 0.018 |
| Subpleural effusion | 11 (50%) | 0 (0%) | 0.00067 |
| Cardiac enlargement | 14 (61%) | 1 (4%) | 0.00075 |
| Bilateral lung disease | 18 (78%) | 14 (61%) | 0.200 |
| Involvement of two or more lobes | 9 (39%) | 9 (39%) | 1.000 |
| Right lung upper lobe | 12 (52%) | 11 (48%) | 0.768 |
| Right lung middle lobe | 15 (65%) | 9 (39%) | 0.077 |
| Right lung lower lobe | 20 (87%) | 16 (70%) | 0.153 |
| Left lung upper lobe | 7 (30%) | 13 (57%) | 0.074 |
| Left lung lower lobe | 17 (74%) | 16 (70%) | 0.743 |
Values are the number and the percentage of patients.
A Patient with Both Heart Failure and COVID-19 Pneumonia
A 64-year-old man with recent travel history to Hubei, China, the epicenter of the COVID-19 outbreak, was admitted to the hospital with fever and orthopnea. He had received a diagnosis of coronary artery disease in the last year and a stent had been placed in his left anterior descending artery. Chest CT (Figure 3) showed multifocal GGO with parenchyma consolidation and subpleural effusion, predominantly involving the upper lobes of the lungs. There was no rounded morphology, while the septal and bronchial walls were thickened. The third swap test for SARS-CoV-2 was positive 1 week after CT scanning.

Computed Tomography of a Patient with Both Heart Failure and COVID-19.
(A) Diffuse disease mixed with ground-glass opacity (GGO) and consolidation in the upper lobes of both lungs, accompanied by interlobular septal thickening and subpleural effusion. (B) Big patchy GGO with irregular morphology in both lungs. Peribronchovascular thickening and subpleural effusion were also found.
Discussion
In this study, the clinical characteristics of heart failure and COVID-19 pneumonia were compared, and the similarities and differences of CT features were also verified. We found that GGO and septal thickening were common lesions in both diseases. Significant differences exist in the distribution type, lesion morphology, upper small pulmonary vein enlargement, fissural thickening, peribronchovascular thickening, subpleural effusion, and cardiac enlargement.
It is essential to identify patients suspected of having COVID-19 as early as possible to control its spread. Contact history, clinical manifestations (fever or respiratory symptoms), laboratory test results (normal or decreased white blood cell count, decreased lymphocyte count), and pulmonary CT findings are included in the evaluation. A patient can be suspected of having COVID-19 if the patient has a contact history and any two of the last three. Even for patients without a clear contact history, the last three can provide evidence for the diagnosis of COVID-19. For patients with heart failure in the absence of fever, chest uncomfortable or chest pain or apnea sometimes occured with respiratory symptoms. In this study, 9 patients in heart failure group had respiratory symptoms. Besides, nearly half of COVID-19 patients may not have fever at admission [7], and the lymphocyte count in patients with heart failure can also be decreased. Although the American College of Radiology recently recommended CT should not be used to screen patients for or as a first-line test to diagnose COVID-19, in our opinion, because of the overlapping or atypical clinical features and the overwhelmed health system, it is reasonable to use pulmonary CT to screen patients suspected of having COVID-19. And usually, without a rapid test kit, CT results will be obtained much more quickly in most countries compared with an RT-PCR test.
In this study, we found that there are some similarities in the imaging features of patients with heart failure and patients with COVID-19 pneumonia. Both diseases had GGO, consolidation, a crazy paving pattern, and septal thickening, which will make it difficult to differentiate heart failure from COVID-19 by CT scanning. However, on careful checking of the images, the distribution types of the two diseases were significantly different. Heart failure was more likely to have a central and gravity-associated gradient distribution, while COVID-19 usually had a more peripheral distribution. Importantly, there were more lesions with rounded morphology in COVID-19 than in heart failure. In detail, although both diseases have septal thickening, heart failure usually has more peribronchovascular thickening, interlobular septal thickening, and fissural thickening, while COVID-19 usually affected the smaller septum. This may be attributed to the different severity of the two diseases at the beginning, which indicates heart failure may progress more rapidly at first. COVID-19 usually progresses with different imaging features at different stages lasting for about 2–3 weeks [8]. At first, GGO is the predominant feature and gradually spreads and becomes consolidated; in the last stage, the consolidation will be absorbed. This study focused only on the initial medical contact, and the CT images shown here almost all belong to the early stage. So predominant GGO and some consolidation were very frequent in COVID-19 in this study. Although fibrous lesions have rarely been reported, five patients with COVID-19 were found with such lesions in this study, which is consistent with current research [9]. Interestingly, the CT characteristics of both diseases may be mixed in a patient with heart failure and COVID-19, or the imaging features of COVID-19 at the initial medical contact will be covered by more progressive heart failure. So it should be more careful to deal with a patient with heart failure in an epidemic area.
Hydrostatic pulmonary edema manifested as interstitial edema and alveolar flooding is the most frequently recognized pathophysiologic and radiologic feature in heart failure [10]. These features (Hydrostatic pulmonary edema) are virtually identical for left-sided heart failure and fluid overload. Interstitial edema occurs first with an increase of 15–25 mmHg in mean transmural arterial pressure and results in the early loss of definition of subsegmental and segmental vessels, mild enlargement of the peribronchovascular spaces, and subpleural effusions. With pressure increasing, alveolar flooding will occur. The pathophysiology of central distribution can be explained by follows: increased tissue hydration allows water to easily flow centrally, the pumping effect of the respiratory cycle causes overall fluid flow toward the hilum, and the contractile property of alveolar septa allows them to expel interstitial infiltrates toward the hilum. Diffuse bilateral interstitial and/or interstitial-alveolar infiltrates are most commonly caused by viruses. Differently from heart failure, COVID-19 is characterized by inflammatory pulmonary edema and alveolar damage. Recently, fibromyxoid exudation, which is absent in heart failure, was found in the lung tissue of a patient obtained at autopsy [11]. All these findings make the imaging different from that in the case of heart failure.
There are several limitations of this study. First, this is a retrospective study with limited cases. Most of the patients with COVID-19 enrolled had nonsevere cases. Secondly, we focused mainly on the clinical and imaging features at the initial medical contact, while the dynamic changes with appropriate therapy are definitely helpful to differentiate the two diseases.
In conclusion, during an epidemic, it is essential for frontline physicians to screen patients suspected of having COVID-19 with CT scanning, which will be very helpful to detect patients at the very beginning and avoid infection of medical staffs as much as possible. Consequently, it is important for physicians to identify the imaging features of COVID-19 pneumonia and heart failure. Although both diseases can have similar GGO and septal thickening, a rounded morphology, a peripheral distribution, and fibrous lesions were relatively specific in COVID-19, while heart failure usually has more peribronchovascular thickening, fissural thickening, subpleural effusion, and cardiac enlargement.
