INTRODUCTION
Tuberculosis (TB) is an airborne infectious disease that threatens public health worldwide. Until the advent of COVID-19, which is caused by the SARS-Cov-2 virus, TB was the leading cause of death due to a single infectious agent worldwide, exceeding that due to HIV/AIDS [1]. It is caused by Mycobacterium, a bacterial genus with a wide spectrum of hosts, and differing host susceptibility and infection pathophysiology [2–4]. To differentiate TB in humans and animals, “human tuberculosis” usually refers to Mycobacterium tuberculosis (MTB) infection, whereas “zoonotic tuberculosis” refers to infections in human and animals mainly caused by a closely related species, Mycobacterium bovis [5,6]. Together these and other TB species comprise Mycobacterium tuberculosis complex (MTBC). Most patients with TB (approximately 90%) are adults, and fewer cases occur in women than men [1]. The World Health Organization (WHO) has estimated that 10 million people (range: 8.9–11.0 million) developed TB in 2019, of which 140,000 had new cases of zoonotic TB (range: 69,800–235,000) [7]. Although the disease has slightly declined in recent years, in 2019 alone, approximately 1.2 million people died from TB, and approximately 500,000 people developed rifampicin-resistant TB [7]. This article discusses the current status of global human and zoonotic TB control and future prospects in the context of COVID-19.
TRENDS IN GLOBAL TB
Human TB control and prevention
The history of human infection with TB dates to 3 million years ago [8]. MTB can cause disease in almost any part of the body, but it primarily invades the respiratory tract. Most patients with TB are diagnosed with active pulmonary TB [9,10]. MTB has infected approximately one-third of the population worldwide [11]. Human TB continues to be among the top ten causes of mortality globally, and it is the leading cause of death due to a single infectious agent [7].
After decades-long neglect in mitigating this disease, renewed global efforts to control TB began in 1991, when the WHO declared TB a major global public health problem [12], which was subsequently declared a global emergency in 1993 [13]. Recommendations were released in 1994 for TB control based on directly observed therapy strategy (DOTS) with a short-course regimen [14]. In response to the 2000 United Nations (UN) Millennium Development Goals, with targets to be reached by 2015, the WHO and the global advocacy organization Stop TB Partnership launched historic firsts with the Global Plan to Stop TB 2001–2005 in 2001 and the Stop TB Strategy 2006–2015 in 2006. Importantly, the latter focused on patient-centered care for all TB-infected individuals, rather than on directly observed therapy with a short-course strategy [15].
To better address the global TB epidemic in the post–Millennium Development Goal era, the UN began developing Sustainable Development Goals (SDGs) in 2012. These goals include a target of a TB-free world by 2030 [16]. The World Health Assembly, in 2014, formulated the End TB Strategy [17], which was launched by the WHO in 2015. The WHO and the Stop TB Partnership also published the Global Plan to End TB 2016–2020: The Paradigm Shift [18], and the UN subsequently released the SDGs in 2015, which became official on January 1, 2016. In 2018 the UN held a high-level meeting to address TB and garner strong political support for strengthening TB control measures in the coming years, as well as to define targets and responsibilities to facilitate the End TB Strategy [19]. Accordingly, in 2019, the WHO and the Stop TB Partnership published an updated version of their global plan, the Global Plan to End TB 2018–2022: The Paradigm Shift: Reaching the United Nations TB Targets [20].
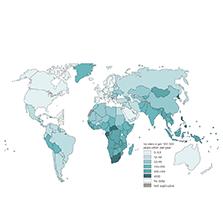
Human TB is a global public health emergency [21]. Globally in 2020, approximately 9.9 million people were estimated to have contracted TB, which is equivalent to 127 cases per 100,000 people (detailed information in Fig 1). The epidemic slightly decreased after 2019, and a slow downward trend has continued since 2000 [1]. Worldwide, an estimated additional 100,000 deaths occurred among HIV-negative people in 2020, compared with 1.2 million deaths in 2019, and an additional 214,000 deaths occurred among HIV-positive people, representing a small increase from 209,000 in 2019 [1]. The global decrease in the absolute number of TB deaths until 2019 was followed by an increase in 2020 in four of the six WHO regions and most of the 30 high-TB-burden countries, because of the COVID-19 pandemic [1]. Risk factors are critical at the population level, including poor working and living conditions, which increase the risk of TB transmission, as well as factors that impair immunity to TB infection and disease (e.g., malnutrition, HIV infection, diabetes, alcohol abuse, smoking, and indoor air pollution) [22]. However, TB continues to be overshadowed by diseases such as HIV, malaria, and now COVID-19 [23].
Advances in human TB treatment
Innovation is crucial for TB control and prevention. The vaccine currently in use was developed in the 1930s, the basic treatment for TB was developed in the 1960s, and the last new anti-TB drug was introduced in many countries approximately three decades ago [24]. The global TB situation is critical, but now is also a time of great promise and discovery for TO BE treatment and prevention [25]. Many substantial changes are on the horizon. For example, the efficacy of M72/AS01E, an adjuvanted protein subunit vaccine, has been demonstrated by clinical trials to prevent the development of active TB in latent TB infection, thus bringing some hope for ameliorating this disease [26].
Furthermore, dramatic changes in the treatment landscape for TB occurred with the introduction of three new drugs and drug regimens over the past decade [27]. For instance, the Nix-TB clinical trial has indicated that an all-oral regimen of bedaquiline, pretomanid, and linezolid (BPaL) has favorable outcomes at 6 months post-treatment, thus suggesting that, if safety management is adequate, the BPaL regimen is a feasible option for patients with highly drug-resistant forms of TB [28]. The BPaL regimen has also been recommend by the WHO for the treatment of multidrug-resistant TB with additional fluoroquinolone resistance [29], thus providing hope for patients with drug-resistant TB.
Finally, system innovations such as digital health technologies are influencing the entire TB patient journey [30]. Digital health and other innovations, if deployed at scale, could help end human TB in the SDG era.
Zoonotic TB control and prevention
Several MTBC organisms, which are present in both animals and the natural environment, can cause zoonotic TB, including M. bovis, M. caprae, M. microti, M. pinnipedii, and M. orygis [31–34]. However, M. bovis is the main causal agent of zoonotic TB in humans [35]. In general, cattle are considered the natural hosts of M. bovis; however, zoonotic TB due to M. bovis and other MTBC pathogens has been reported in other species of domesticated animals and wildlife, and remains a major zoonosis [36]. The most common pathways of transmission to humans are inhalation, consumption of unpasteurized milk, and close contact with infected animals or untreated animal products [37]. For example, in recent years, M. bovis has been confirmed in pastoralists in Nigeria [38]. Reverse zoonoses due to M. tuberculosis, which is transmitted from humans to goats, pigs, and cattle, have also been reported in Nigeria [39], owing to close human and animal contact in most pastures, factories and communities in the country.
After the WHO recognized the implications of zoonotic TB to public health in 1950, TB in animals has been controlled and nearly eliminated in several developed countries but in only very few low- and middle-income countries [40], where zoonotic TB has substantial economic effects and can simultaneously affect the health of humans, livestock, and ecosystems [41]. This threat of zoonotic TB spurred development of a resolution in 1983 by the World Organization for Animal Health, or OIE (formerly the Office International des Epizooties), calling for eradication of M. bovis for both public health and economic reasons [42]. In view of the concerns regarding zoonotic TB in humans and animals due to M. bovis infection, in November 1993, the WHO convened a meeting on zoonotic TB in Geneva and proposed a project protocol to further examine the zoonotic features of bovine TB [43]. In the past decade, zoonotic TB has attracted new attention from international health authorities, such as the WHO, the Food and Agriculture Organization (FAO), and OIE [44]. The WHO and Stop TB Partnership’s Global Plan to End TB 2016–2020: The Paradigm Shift first included communities and people at risk of contracting zoonotic TB as a key population [18]. In October 2017, the WHO, FAO, and OIE developed the first roadmap for efforts against zoonotic TB under the One Health (i.e., animal, human, and environmental health) umbrella, which was launched at the 48th Union World Conference on Lung Health that year [45].
A recent study has noted that zoonotic TB is reemerging as an infectious disease in high-income countries and as a neglected disease in low- and middle-income countries [46]. Furthermore, because the burden of M. bovis–associated zoonotic TB is unknown, it is likely to be underestimated [47]. The prevalence estimates of zoonotic TB are also inaccurate, because current laboratory tests cannot distinguish the species of MTBC infecting humans or animals [48]. The WHO has estimated the zoonotic TB burden according to scientific studies since 2016 [49] and has proposed strengthening the surveillance of zoonotic TB to more accurately determine the disease burden. Of the 10 million people in 2019 with new cases of active TB, 140,000 (range: 69,800–235,000) have been estimated to have zoonotic TB (1.4%), and approximately 11,400 (range: 4,470–21,600) ultimately died (8.1%) [7]. For zoonotic TB in cattle, studies have reported a prevalence of confirmed M. bovis zoonotic TB ranging from 0% to 28%; however, some of the culture methods and the array of molecular methods currently used in laboratories are inappropriate for the diagnosis of zoonotic TB [50].
Concerns regarding zoonotic TB, as reported for decades, still remain valid [51,52]. Post-mortem examination and the single intradermal comparative cervical tuberculin test are the major diagnostic tools for bovine TB [53]. However, these tests have biosafety issues, are time-intensive, and lack both political commitment and high-quality surveillance data. Together, these hurdles have contributed to an increase in TB incidence worldwide [46]. To address this challenge, efforts are underway to adapt human TB diagnostics to detect potentially zoonotic TB organisms in cattle [54]. However, because M. bovis cannot be eradicated from livestock while continued transmission occurs between domestic animals and wildlife [55], controlling M. bovis infection with detect and cull policies remains the backbone of zoonotic TB risk reduction. Animal vaccination is also proving beneficial in certain circumstances. Accordingly, oral bacillus Calmette-Guérin vaccine should be administered to animals at large scale as a complement to traditional control measures to induce protection against TB and decrease host reservoirs [56,57]. An even more troubling prospect involves animal carriers of drug-resistant MTB contributing to reverse zoonosis at the human-animal interface [58]. Despite these concerns, the zoonotic TB in humans, compared with other diseases, might have received a disproportionately low allocation of scientific attention and resources in recent years [52].
Effects of the COVID-19 pandemic
The pandemic has created unprecedented global socioeconomic disruption [59]. Its influence on TB control is likely to extend worldwide, particularly in terms of case detection and short-term TB mortality [60]: the number of TB cases is projected to increase by 6.3 million in the next 5 years, together with a 20% increase in deaths from TB in the same period [61,62], thus delaying achievement of the WHO End TB target.
Before COVID-19, a large decline had been observed globally in the number of new human TB diagnoses and reports, from 7.1 million in 2019 to 5.8 million in 2020. The numbers returned to 2012 levels after an 18% decline, far below the approximately 10 million TB cases in 2020 [1]. In China, for example, a marked decrease in case notifications was associated with COVID-19 interventions: in the 11 weeks during and immediately after the COVID-19 lockdown, the case notification rate was 20% lower than that in the corresponding period in 2019 [63]. Similar findings have been reported in other countries [64–66]. Empirical evidence regarding the long-term effects of the pandemic on TB outcomes has been limited to date, and further study is required [67].
FUTURE PROSPECTS
Achieving a TB-free world is a desirable goal with respect to human, animal, and environmental health—according to the tenets of One Health. Given the interspecies transmission of MTBC through close human-animal interaction, human TB cannot be eradicated without addressing the issue of zoonotic TB. The 2020 progress report from the UN Secretary-General recommended ten priority actions to accelerate advancement toward the global TB targets; in addition, the roadmap developed by the WHO, FAO, and OIE proposes ten priorities to address zoonotic TB. Both call for high-level leadership, multisectoral and collaborative action, greater investment in high-quality scientific research and innovation, and universal health coverage for every patient with TB. The WHO, civil society, including Stop TB Partnership, the UNION, the Global Fund, etc., have proposed a strong call to action for greater access to TB preventive treatment, urging governments to support research and innovation, particularly in vaccine development, to better fight against TB and to ensure that at least 30 million people receive TB preventive treatment by 2022 [68].
Although the COVID-19 pandemic has posed severe challenges to global TB control, it also has brought unique opportunities for developing innovative approaches to ensure patient-centered diagnosis, treatment, and management. TB health systems must avoid disruption and provide services under all circumstances, and must have mechanisms to restore routine services in global emergencies, such the COVID-19 pandemic. Governments and health systems worldwide must immediately take ambitious and radical action to integrate the innovations in service delivery developed in response to the COVID-19 pandemic into the current health system, and to use these new methods to accelerate progress toward the End TB Strategy targets.