INTRODUCTION
Extracellular vesicles (EVs) include three major and distinct groups of small membrane-enclosed packets that contain bioactive molecules secreted by all cells. Different EVs arise from distinct biogenesis schemes [1–3]. Exosomes, or small EVs, range in size from 50 to 200 micrometers, and are derived from intraluminal vesicles within multivesicular compartments in the endocytic pathway of cells; microvesicles range in size from 100 to 1,000 micrometers, and are formed by plasma membrane budding; apoptotic bodies range in size from 100 to 5,000 micrometers, and are formed by the disintegration of cells [4]. Each subtype of EVs should be expected to have unique molecular characteristics and functions; however, standard protocols to isolate EVs according to their size and density are widely recognized to be inadequate in yielding homogeneous populations of each sub-type. The recognition of this limitation in the isolation of EVs led the International Society for EVs to recommend the use of “EV” as a broad classifier term for these types of vesicles [5,6] Exosomes are often referred to as vesicles belonging to the group of small EVs [7], the term used herein. However, understanding the composition of EVs, the factors that influence the loading of EVs, and their functions are topics of considerable interest [3,8]. Many studies on the composition of small EVs have shown an enrichment in defined membrane and cytosolic molecules from the endocytic pathway [5,9]. Membrane proteins in small EVs include MHC class II complexes [5,9], members of the tetraspanin family (CD9, CD63, and CD81), endosomal sorting complex required for transport (ESCRT), and integrins [10,11]; membrane-associated molecules including Rab GTPases (Rab4, Rab11, and Rab27) [12,13]; cytoskeletal proteins including actin, tubulin, and cofilin; and cytosolic proteins including ALG-2-interacting protein X (Alix), tumor susceptibility gene 101 (TSG101), and heat shock proteins (e.g., Hsp70 and Hsp90). In addition, nucleic acids, including DNA, coding and non-coding RNAs (mRNA, miRNA, circRNA, and tRNA), and several categories of RNA binding proteins have been identified within the lumens of small EVs [14–17]. Together, these molecules can initiate, enhance, or inhibit cellular functions after the delivery of EVs to recipient cells.
The functional role of EVs was discovered in several types of studies, including those on the development of immune responses [18,19] and those valuating the therapeutic potential of adoptively transferring pluripotent cells to reconstitute impaired cellular functions [20,21] or stromal cells [22]. Examples include studies evaluating the therapeutic effects of the transfer of mesenchymal stems cells (MSCs) to mouse recipients, in which factors derived from these cells and secreted into the MSC-conditioned medium have been found to have immunomodulatory, proangiogenic, and tissue-tropic activities without need of the presence of a mother cell that releases these factors [23]. Similar observations have been made in studies on the potential of stromal cells to protect against tubular injury in a cisplatin-induced acute renal failure model [22]. Conditioned medium from cultured stromal cells induces the migration and proliferation of kidney-derived epithelial cells. Moreover, intraperitoneal administration of this conditioned medium to mice injected with cisplatin has been found to diminish tubular cell apoptosis, increase survival, and limit renal injury. Together, these studies have demonstrated that the cells’ secretome in the conditioned medium can serve as a cell-free therapeutic agent for treating inflammatory diseases [21].
Studies on the molecular composition of EV membranes
Like cells, EVs display molecules on their surfaces that interact with cells and other surfaces in tissues. Molecules on the surfaces of EVs include lipids, such as phosphatidylserine (PS), sphingomyelin, cholesterol, and ceramides; carbohydrate moieties [24,25], such as glycosphingolipid glycan groups [26,27]; and many types of membrane proteins. Some of these lipids are enriched in exosomes with respect to their parental cells, on the basis of lipid data from several studies [24]. Specifically, cholesterol, sphingomyelinases, glycosphingolipids, and PS have been found to be enriched by twofold to threefold. These lipids mediate binding of exosomes to recipient cells. For example, PS binds the T-cell immunoglobulin- and mucin-domain-containing molecule (TIM)-1 and TIM-4 immunomodulatory receptors on phagocytic cells [28], thus promoting selective interaction and uptake of EVs that express this molecule. Notably, the observed lipid content of EVs can provide information regarding the purity of the preparation. Specifically, the presence of cardiolipin may suggest contamination of the preparation with lipids from internal organelles such as mitochondria [24]. EVs are also enriched in a variety of membrane proteins. Some of these proteins include selected cytokines and chemokines such as IL-8 and chemokine (C-X-C motif) ligand 1 (CXCL1) [29]. Interestingly, cytokine incorporation in EVs, including expression on the EV membrane is cell/tissue origin dependent [29]. For example, most cytokines secreted by CD4+ T cells are within EVs, whereas placental villous tissue preferentially secretes cytokines in free (soluble) form. Other surface molecules on EVs include the major histocompatibility antigens and accessory molecules necessary for antigen specific activation of both CD4+ and CD8+ T cells [30]. The display of these molecules on EVs is also cell source dependent, as convincingly demonstrated by studies on dendritic cells, in which EVs from mature DCs are relatively enriched in CD86 and intercellular adhesion molecule 1 (ICAM-1), whereas EVs from immature DCs are enriched in milk fat globule–epidermal growth factor–factor VIII (MFG-E8) [31].
The understanding of how molecules are selected for packaging in EVs is incomplete. Insight regarding the selection mechanisms influencing the presence of proteins in the delimiting membranes of EVs and the consequences of the differential expression of these proteins on EV function has been provided by studies on tetraspanins and integrins. Tetraspanins are small transmembrane proteins that function in cell migration, signal transduction, and intracellular trafficking. Of the 33 tetraspanins in the human genome, several have been shown to localize to small EVs [32–34]. These tetraspanins are often used as specific markers of exosomes [34,35]. Integrins are cell adhesion receptors that bind the extracellular matrix, cell surface, and soluble ligands. They are transmembrane alpha-beta heterodimers, and at least 18 alpha and eight beta subunits, generating 24 heterodimers, are known in humans [36]. The functions of integrins are partly dependent on some integrins being limited to specific cell types or tissues, and by the pairing of alpha and beta subunits determining their ligand-binding specificity (reviewed in [36]. Therefore, the differential expression of tetraspanins and integrins on EVs might be expected to influence EV interactions and functions. Studies by Zöller and colleagues have provided extensive insight into the interactions of tetraspanins and integrins in EVs [37,38]. One study has focused on interactions between tetraspanin 8 and integrin beta 4 in EV lysates from rat tumor cell lines [39]. The levels of tetraspanin 8 in the tumor lines were found to be modulated by the introduction of overexpression constructs and by knockdown of their protein levels. Moreover, the abundance of tetraspanins and integrins on EVs was affected by changes in the expression of these molecules in the parental cells from which these EVs were derived. In co-immunoprecipitation experiments, tetraspanins and integrins have been found to promiscuously engage in interactions as well as in several preferential interactions. For instance, Tetraspanin 8 interacts with the alpha4 and beta4 integrin chains, thus also affecting the pairing of integrins in the resultant EVs. Notably, several apparently subtle changes in the molecular interactions of tetraspanins and integrins have been found to alter the binding of EVs with cells and their tropism in vivo. Those observations have complemented findings from an earlier study indicating that only small EVs expressing a defined set of tetraspanins and associated molecules target endothelial cells [40]. Moreover, interactions of EVs with hematopoietic cells, for example, that result in EV binding and uptake have been found to be favored by the expression of several adhesion molecules including CD54 [39]. Regarding EV tropism, small EVs expressing tetraspanin 8 are more readily taken up by the pancreas and lung but rarely taken up by the liver and gut. Together, those studies and others have provided strong evidence indicating that alterations in the abundance and identity of tetraspanin and integrins on small EVs affects the molecular pairings on the small EV surface and consequently their functional interactions in vivo.
EV membrane composition is modulated by intracellular infection
Intracellular infection of cells also modulates the composition of adhesion molecules expressed by EVs released by infected cells. For example, in infection of cells by Epstein-Barr virus, the EV content, including the expression of adhesion molecules, is modulated by Latent Membrane Protein 1 (LMP-1) [41]. EVs isolated from the mouse macrophage cell line RAW264.7 infected with the intracellular pathogens Leishmania donovani or Salmonella Typhimurium have been isolated and subjected to proteomic analysis [42,43]. Analysis of integrins in the EV preparations has shown that S. Typhimurium infection alters the levels of specific integrins (Tables 1 and 2). For example, S. Typhimurium infection of RAW264.7 cells leads to a decrease in integrin subunit alpha 4 (ITGA4), integrin subunit alpha M (ITGAM), integrin subunit beta 1 (ITGB1), and integrin subunit beta 2 (ITGB2) in small EVs at 24 hours post-infection (hpi) (Table 1). However, at 48 hpi, the S. Typhimurium infection leads to an increase in integrin subunit alpha M (ITGAM) and integrin subunit beta 2 (ITGB2) (Table 2). Tetraspanin expression in EVs is also modulated by S. Typhimurium infection (Table 3).
Integrins in small EVs from Salmonella-infected RAW264.7 macrophages isolated at 24 hpi (MOI 5:1).
| Symbol | Gene name | Accession number | Fisher’s exact test (p-value) | Expression fold change (24 hpi vs. control) |
|---|---|---|---|---|
| ITGA4 | integrin subunit alpha 4 | Q792F9 | 0.0001 | −5 |
| ITGA5 | integrin subunit alpha 5 | P11688 | 0.78 | 1.7 |
| ITGAM | integrin subunit alpha M | E9Q604 | 0.00055 | −2.5 |
| ITGAV | integrin subunit alpha V | P43406 | 0.38 | −2.5 |
| ITGB1 | integrin subunit beta 1 | P09055 | 0.0001 | −3.333 |
| ITGB2 | integrin subunit beta 2 | P11835 | 0.0001 | −3.333 |
| ITGB7 | integrin subunit beta 7 | P26011 | 0.38 | −2.5 |
Integrins in small EVs from Salmonella-infected RAW264.7 macrophages isolated at 48 hpi (MOI 5:1).
| Symbol | Gene name | Accession number | Fisher’s exact test (p-value) | Expression fold change (48 hpi vs. control) |
|---|---|---|---|---|
| ITGAM | integrin subunit alpha M | E9Q604 | 0.025 | 1.8 |
| ITGB2 | integrin subunit beta 2 | P11835 | 0.04 | 1.6 |
| ITGA4 | integrin subunit alpha 4 | Q792F9 | 0.089 | −1.429 |
| ITGA5 | integrin subunit alpha 5 | P11688 | 0.13 | 4 |
| ITGB1 | integrin subunit beta 1 | P09055 | 0.52 | 1 |
| ITGAV | integrin subunit alpha V | P43406 | 0.74 | 1.4 |
| ITGB7 | integrin subunit beta 7 | P26011 | 0.85 | 1 |
Tetraspanins in small EVs from Salmonella-infected RAW264.7 macrophages isolated at 24 hpi (MOI 5:1).
| Symbol | Gene name | Accession number | Fisher’s exact test (p-value) | Expression fold change (24 hpi vs. control) |
|---|---|---|---|---|
| CD81 | CD81 molecule | P35762 | 0.00029 | −5 |
| CD82 | CD82 molecule | P40237 | 0.72 | −1.111 |
| CD9 | CD9 molecule | P40240 | 0.084 | −1.429 |
| TSPAN14 | tetraspanin 14 | Q8QZY6 | 0.14 | −5 |
The pattern of adhesion molecule expression on EVs determines tissue tropism
As discussed earlier, EVs contain a range of molecules in their delimiting membranes, including lipids, carbohydrates, and proteins, which are likely to affect the cellular interactions of EVs and tropism. Although the molecules found in EVs are constrained by the molecular composition of their parental cells, EVs do not necessarily contain the most abundant molecules in their parental cells [44,45]. This phenomenon suggests the existence of a selective mechanism for loading of specific proteins into EVs that can be modulated to achieve expression of the desired molecular profile. EV interactions with cells have been hypothesized to mirror the interactions of their parental cells, and EVs may preferentially home to tissues containing their parental cells. Lyden and colleagues have contributed several studies on this topic [46–48]. In one study, small EVs were recovered from tumor lines with known metastatic destinations. Small EVs from the BxPC-3 and HPAF-II lines showed up to fourfold greater accumulation in the liver than other organs, in agreement with the tissue tropism of those tumor lines. The small EVs from MDA-MB-231, compared with small EVs from the tumor lines described above, exhibited a more than threefold accumulation in the lungs. Moreover, 831-BrT EVs efficiently localized to the brain, showing a more than fourfold increase with respect to small EVs from two other tumor lines not known to metastasize to the brain [46]. The molecules likely to be primarily responsible for targeting the EVs to specific cells or organs are tetraspanins and integrins. Quantitative mass spectrometry of small EVs from these metastatic tumor lines has identified six integrins among the top 40 most abundant adhesion molecules. For example, integrin alpha 6 (ITGα6) and its partners ITGβ4 and ITGβ1 are abundant in lung-tropic EVs. In contrast, ITGβ5, which associates only with ITGαv has been detected primarily in liver-tropic EVs. The critical requirement for EV-based ITGβ4 in lung tropism has been further confirmed by knock-down of ITGβ4. In the recipient tissues, EVs associated with molecules such as laminin and fibronectin have been found to activate Src phosphorylation and pro-inflammatory S100 gene expression [46]. In another study, melanoma B16BL6 cell-derived small-EVs have been found to localize to the lungs within 10 minutes after injection of the EVs into animals. Treatment of EVs with proteinase K, thus decreasing the vesicular integrin α6β1 displayed on the EVs, has been found to decrease the distribution to the lungs [49]. Finally, EVs with pronounced gut-homing properties express integrin α4β7 [50]. T cells induced by retinoic acid secrete EVs with higher expression of the integrins α4β7. These EVs suppress the expression of some adhesion molecules in the gut, thus limiting subsequent lymphocyte homing [50]. Together, these studies support the hypothesis that integrin selection and display on EVs play critical roles in EV homing.
Studies on small EV transfer into hosts
Studies seeking to determine the fate of EVs administered to recipient hosts have reported mixed results. Many studies have used various approaches to label EVs to track their entry and retention into tissues [51,52]. Some labeling approaches have included direct loading with dyes such as PKH26/27, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, or 4-chlorobenzenesulfonate salt (DiD)-lipophilic dye, or genetic engineering of proteins in EVs with luminescent probes such as Renilla or Gaussia luciferases [53,54]. Most of those studies have evaluated the administration of heterologous EVs, compared with autologous EVs, by using varying routes of delivery that influence the kinetics and biodistribution of small EVs. The picture that has emerged from those studies is quite mixed. Smyth and colleagues have found rapid clearance and minimal tumor accumulation of intravenously injected unmodified tumor derived small EVs [55]. This finding may suggest that the unique protein and lipid composition of small EVs does not appreciably influence their bio-distribution. Nonetheless, the study found that innate immune mechanisms in the host affects the biodistribution of small EVs. In a recent publication, Kang and colleagues have compiled and analyzed more than 29,000 reports on the biodistribution of small EVs published until June of 2019 [56]. The conclusions of this analysis agreed with the observations from other researchers in that the site of EV retention after the transfer appears to be dependent on several factors including the route of administration [intravenous vs. intraperitoneal vs. subcutaneous], the number of particles transferred, the time at which the transfers were evaluated, and the recipient animals. Among the insightful observations from the analysis in reference [49], EVs were found to be quickly cleared from the blood within 30 minutes after their administration. In most studies, the liver, followed by the lungs, have been the most reproducible accumulation sites of EVs. Other tissues in which EVs are retained are the spleen, GI tract, and kidney, in that order. The route of administration of EVs influences the site of EV retention. A study by Wiklander and colleagues [57], included in the analysis by Kang et al. [49], has evaluated the biodistribution of EVs prepared from three cell sources. Among the observations, greater [56] trafficking of EVs to the pancreas and GI tract was observed when EVs were administered subcutaneously or intraperitoneally, whereas preferential accumulation of EVs from the same source in the liver and spleen was observed when the EVs were administered intravenously. Specifically, EVs derived from C2C12, a muscle-derived cell line, showed more significant liver accumulation than EVs from B16F10, a melanoma-derived line, or from bone marrow-derived dendritic cells. The EVs from the melanoma line preferentially accumulated in the GI tract. Other studies, including those in an acute kidney injury model [58], have shown that EVs from mesenchymal stem cells accumulate in injured kidneys. Hui and colleagues have observed preferential accumulation of EVs prepared from Salmonella-infected macrophages in the GI tract and lungs, which was more significant than the dye accumulation in these organs [59]. The accumulation of EVs in the study by Hui and colleagues was also dependent on the route of EV administration [59]. Together, these findings indicate that the route of administration of EVs affects the biodistribution in hosts. The analysis also underscored the effects of cell sources on the tissue destination of EVs.
Factors affecting the homing of EVs
Marked differences exist in the biodistribution of EVs from normal cells or tissues vs. those derived from immortalized cells. Garofalo and colleagues have generated EVs from murine lung and colon cancer lines; a human lung cancer cell line, and human liver biopsy samples from healthy individuals. The results indicated that tumor-derived EVs, but not EVs derived from healthy tissue, showed selective accumulation of fluorescence at the tumor site 24 h after injection [51]. Moreover, trafficking to, and retention in, tumors has been reproducibly observed with EVs derived from immortalized cells, even if their parental cells are not derived from that tumor. The tumor tropism of these EVs has been further demonstrated in a study in which human EVs were found to be able to target mouse mammary tumors [51], thus suggesting that damaged tissues (such as tumors) can recruit EVs derived from heterologous sources. Processes within the host appear to affect the distribution of small EVs. Smyth and colleagues have shown that intravenously administered unmodified EVs have a short half-life and are rapidly taken up by the mononuclear phagocyte system, particularly in the liver, lungs, and spleen, thus leading to minimal accumulation in the intended tissues and undesired delivery to unintended tissues [55]. Their studies have suggested that the innate immune system, with help from complement opsonization, contributes to removing tumor-derived EVs from circulation. Other observations by Smyth and colleagues [55] on the preferential trafficking of EVs to tumors complement the observations in several other studies [60]. Hypoxic conditions have been shown to stimulate greater release of small EVs by tumor cells [61,62]. Hypoxic conditions also increase the effects of EVs in recipient tissues by enhancing processes such as angiogenesis. However, the mechanism underlying the preferential recruitment of administered small EVs to tumors is poorly understood. EVs derived from specific cells express surface antigens, including CD47, thus limiting their uptake by monocytes, and resulting in extended blood circulation times and an enhanced opportunity to circulate widely [63–65]. The absence of such molecules from EVs derived from normal cells or tissues may explain why evidence of directed homing of “normal” EVs is challenging to find. “Damaged” tissues, such as tumors, have been suggested to display greater expression of molecules such as CD54 [39] or S100, which are proteins found in lymph nodes in patients with melanoma and are predictors of poor prognosis [66].
Strategies to reliably direct EV homing
The desire to exploit predictable EV distribution has been approached in several studies. Zhang and colleagues have explored monocyte membrane-decorated MSC small EVs to improve the specific targeting capability of these EVs to injured hearts and promote heart repair in a myocardial ischemia-reperfusion injury model in mice [67]. Monocyte membranes were isolated from the murine monocyte-macrophage cell line, RAW264.7 cells, whereas small EVs were derived from MSCs. The small EVs and macrophage-derived membranes were fused through a fusion and extrusion method. These monocyte membrane-decorated small EVs exhibited enhanced targeting efficiency to injured myocardium.
Peptides might also be important in the localization of EVs to specific compartments. In a proof of principle, small EVs have been engineered to increase their targeting capabilities by including targeting peptides fused to the extracellular regions of proteins displayed on these EVs; for example, the extracellular N-terminus of Lamp2b is present on EVs [68]. This principle has been used to target EVs to the central nervous system, where the rabies viral glycoprotein (RVG) peptide (YTIWMPENPRPGTPCDIFTNSRGKRASNG) fused to Lamp2b specifically binds acetylcholine receptor 3 and targets dendritic cell-derived EVs to the brain after intravenous injections [68]. T7 peptide can also be fused to Lamp2b to target EVs to the brain. In comparison, to control EVs, T7-containing EVs have been shown to have enhanced delivery efficiency to the brain in a model of glioblastoma [69]. Moreover, muscle-specific peptide fused with Lamp2b has been used to successfully deliver EVs to muscles [70].
CONCLUSIONS/PERSPECTIVES
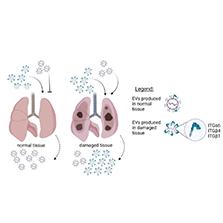
A review of the biology of EVs regarding their homing/tropism has revealed the following observations: [1] More EVs are released from cells under stressful conditions, such as immortalization (malignancy), hypoxia, or pathogen infection, than from normal cells from undamaged tissues. [2] EVs released under stressful conditions express higher levels of adhesion molecules, including tetraspanins and integrins (Fig 1). [3] The parental cell’s tissue origin predetermines the identities of the tetraspanins and integrins expressed on EVs. [4] EVs released by cells under stressful conditions express molecules such as CD47 (“do not eat me” signals) that prevent EV uptake by phagocytic cells, thus delaying their clearance from circulation. [5] Tissues undergoing stressful processes, including injury from malignant growth, express adhesion molecules that render these tissues more receptive to EVs (Fig 1). Normal tissues are poor targets for administered EVs.

Homing patterns of EVs are determined by their characteristics. Cells in damaged tissues, such as damaged lung, produce more EVs (including EVs with higher expression of adhesion molecules) and are more likely to take up heterologous EVs than cells in normal, non-damaged tissues. The EVs preferentially retained by/targeted to lungs contain specific surface molecules, such as ITGα6, ITGβ4, or ITGβ1.
Efforts to modify EVs to improve their reproducible tropism have been promising. Those efforts will be supported by more studies on the molecular composition of homogeneous preparations of EVs. Developing representative immortalized lines from each tissue should be a worthwhile undertaking. Experimental designs informed by a realistic understanding of EV biology are likely to deliver promising translatable findings.