- Record: found
- Abstract: found
- Article: not found
XIAP-mediated Caspase Inhibition in Hodgkin's Lymphoma–derived B Cells
Read this article at
Abstract
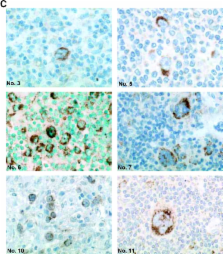
The malignant Hodgkin and Reed-Sternberg cells of Hodgkin's lymphoma (HL) and HL-derived B cell lines were previously shown to be resistant to different apoptotic stimuli. We show here that cytochrome c fails to stimulate caspases-9 and -3 activation in cytosolic extracts of HL-derived B cells, which is due to high level expression of X-linked inhibitor of apoptosis (XIAP). Coimmunoprecipitation studies revealed that XIAP, apoptosis protease-activating factor–1, and caspase-3 are complexed in HL-derived B cell lysates. Even after stimulation with exogenous cytochrome c and dATP, XIAP impairs the proteolytic processing and activation of caspase-3. In cytosolic extracts, inhibition of XIAP by the second mitochondria-derived activator of caspases (Smac)/DIABLO, or immunodepletion of XIAP restores cytochrome c–triggered processing and activation of caspase-3. Smac or a Smac-derived agonistic peptide also sensitized intact HL-derived B cells for the apoptotic action of staurosporine. Finally, Hodgkin and Reed-Sternberg cells of primary tumor HL tissues also constitutively and abundantly express XIAP. The results of this paper suggest that high level XIAP expression is a hallmark of HL, which may play a crucial role in resistance to apoptosis.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade.
- Record: found
- Abstract: not found
- Article: not found
IAP family proteins--suppressors of apoptosis.
- Record: found
- Abstract: found
- Article: not found
