- Record: found
- Abstract: found
- Article: found
Beneficial effect of butyrate‐producing Lachnospiraceae on stress‐induced visceral hypersensitivity in rats
Read this article at
Abstract
Background and Aim
Emerging evidence indicates that psychological stress is involved in the pathogenesis of irritable bowel syndrome, which is characterized by visceral hypersensitivity and may be accompanied by gut dysbiosis. However, how such stress contributes to the development of visceral hypersensitivity is incompletely understood. Here, we aimed to investigate the influence that stress‐induced microbial changes exert on visceral sensitivity, as well as the possible underlying mechanisms associated with this effect.
Methods
Male Sprague–Dawley rats underwent chronic water avoidance stress (WAS) to induce visceral hypersensitivity. Visceral sensitivity, colonic tight junction protein expression, and short‐chain fatty acids of cecal contents were measured. Fecal samples were collected to characterize microbiota profiles. In a separate study, oral gavage of Roseburia in WAS rats was conducted to verify its potential role in the effectiveness on visceral hypersensitivity.
Results
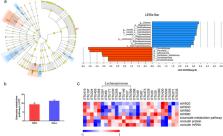
Repeated WAS caused visceral hypersensitivity, altered fecal microbiota composition and function, and decreased occludin expression in the colon. Stressed rats exhibited reduced representation of pathways involved in the metabolism of butyrate and reduced abundance of several operational taxonomic units associated with butyrate‐producing bacteria, such as Lachnospiraceae. Consistently, supplementation with Roseburia hominis, a species belonging to Lachnospiraceae, significantly increased cecal butyrate content. Moreover, Roseburia supplementation alleviated visceral hypersensitivity and prevented the decreased expression of occludin.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: found
Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease
- Record: found
- Abstract: found
- Article: not found
The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection.
- Record: found
- Abstract: found
- Article: not found