- Record: found
- Abstract: found
- Article: found
Development of oral immunomodulatory agents in the management of multiple sclerosis
Abstract
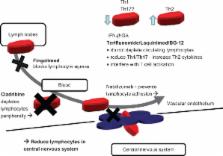
The emergence of oral disease-modifying therapies in multiple sclerosis (MS) will have a significant impact on the evolving scenario of immunomodulatory treatments in MS where current therapies are all injectable. Reducing relapses in trials translates for individuals with MS into a therapeutic aim of stopping future events. Thus the possible absence of any perceived benefits to the individual together with the long disease course, variable outcome, and a younger age group affected in MS makes side effects the major issue. The use of disease-modifying therapies as a whole needs to be placed in the context of a widening therapeutic indication where the use of these therapies is being justified at an increasingly early stage and in pre-MS syndromes such as clinically isolated and radiologically isolated syndromes where no fixed disability is likely to have accumulated. The five oral therapies discussed (cladribine, fingolimod, laquinimod, BG-12, and teriflunomide) have just completed Phase III studies and some have just been licensed. New oral drugs for MS need to be placed within this evolving marketplace where ease of delivery together with efficacy and side effects needs to be balanced against the known issues but also the known long-term safety of standard injectables.
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Recombinant expression of N-terminal truncated mutants of the membrane bound mouse, rat and human flavoenzyme dihydroorotate dehydrogenase. A versatile tool to rate inhibitor effects?
- Record: found
- Abstract: found
- Article: not found
Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study.
- Record: found
- Abstract: found
- Article: not found