- Record: found
- Abstract: found
- Article: found
Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients
Read this article at
Abstract
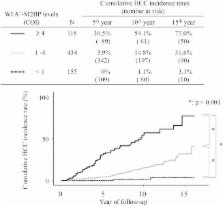
The Wisteria floribunda agglutinin-positive human Mac-2-binding protein (WFA +-M2BP) was recently shown to be a liver fibrosis glycobiomarker with a unique fibrosis-related glycoalteration. We evaluated the ability of WFA +-M2BP to predict the development of hepatocellular carcinoma (HCC) in patients who were infected with the hepatitis C virus (HCV). A total of 707 patients who had been admitted to our hospital with chronic HCV infection without other potential risk factors were evaluated to determine the ability of WFA +-M2BP to predict the development of HCC; factors evaluated included age, sex, viral load, genotypes, fibrosis stage, aspartate and alanine aminotransferase levels, bilirubin, albumin, platelet count, alpha-fetoprotein (AFP), WFA +-M2BP, and the response to interferon (IFN) therapy. Serum WFA +-M2BP levels were significantly increased according to the progression of liver fibrosis stage ( P < 0.001). In each distinctive stage of fibrosis (F0-F1, F2, F3, and F4), the risk of development of HCC was increased according to the elevation of WFA +-M2BP. Multivariate analysis identified age >57 years, F4, AFP >20 ng/mL, WFA +-M2BP ≥4, and WFA +-M2BP 1-4 as well as the response to IFN (no therapy vs. sustained virological response) as independent risk factors for the development of HCC. The time-dependent areas under the receiver operating characteristic curve demonstrated that the WFA +-M2BP assay predicted the development of HCC with higher diagnostic accuracy than AFP. Conclusion: WFA +-M2BP can be applied as a useful surrogate marker for the risk of HCC development, in addition to liver biopsy. (H epatology 2014;60:1563–1570)
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Management of hepatocellular carcinoma.
- Record: found
- Abstract: found
- Article: not found
Time-dependent ROC curves for censored survival data and a diagnostic marker.
- Record: found
- Abstract: found
- Article: not found