- Record: found
- Abstract: found
- Article: found
Protein succinylation: regulating metabolism and beyond
Read this article at
Abstract
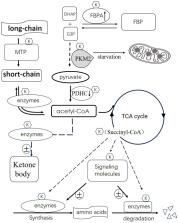
Modifications of protein post-translation are critical modulatory processes, which alters target protein biological activity,function and/or location, even involved in pathogenesis of some diseases. So far, there are at least 16 types of post-translation modifications identified, particularly through recent mass spectrometry analysis. Among them, succinylation (Ksuc) on protein lysine residues causes a variety of biological changes. Succinylation of proteins contributes to many cellular processes such as proliferation, growth, differentiation, metabolism and even tumorigenesis. Mechanically, Succinylation leads to conformation alteration of chromatin or remodeling. As a result, transcription/expression of target genes is changed accordingly. Recent research indicated that succinylation mainly contributes to metabolism modulations, from gene expression of metabolic enzymes to their activity modulation. In this review, we will conclude roles of succinylation in metabolic regulation of glucose, fat, amino acids and related metabolic disease launched by aberrant succinylation. Our goal is to stimulate extra attention to these still not well researched perhaps important succinylation modification on proteins and cell processes.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function.

- Record: found
- Abstract: found
- Article: found
CPT 1A‐mediated succinylation of S100A10 increases human gastric cancer invasion

- Record: found
- Abstract: found
- Article: found
