- Record: found
- Abstract: found
- Article: found
Staging and Response Evaluation to Neo-Adjuvant Chemoradiation in Esophageal Cancers Using 18FDG PET/CT with Standardized Protocol
Read this article at
Abstract
Background:
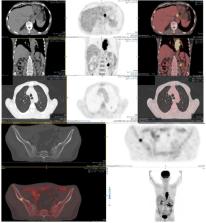
Precise staging of esophageal cancer (EC) is important for selection of optimal treatment option and prognostication. Aim of this study was to assess the role of 18FDG PET/CT in staging and response evaluation to neoadjuvant chemoradiation (nCR) in EC patients using standardized imaging protocol.
Material and methods:
This prospective study was conducted at PET/CT Section of Department of Radiology, Aga Khan University Hospital Karachi, Pakistan from July 2017 till February 2018. We included 34 biopsy proven EC patients who had 18FDG PET/CT and CT of neck, chest and abdomen as part of initial staging. Eleven patients had post-nCR 18FDG PET/CT using standardized imaging protocol as per EANM guidelines. CT and PET/CT based staging was compared. Based on PERCIST criteria, response evaluation was assessed using change in highest SUVmax (%∆SUVmax) in baseline and follow-up scans (primary lesion, node or extra-nodal metastases).
Results:
Mean age of cohort was 57 ± 14 years (23 males and 11 females) having adenocarcinoma (AC) in 23 and squamous cell cancer (SCC) in 11 patients. Mean 18FDG dose, uptake time and hepatic SUVmean for baseline scans were 169 ±54 MBq, 65 ±10 minute and 1.91 ± 0.49 which were within ± 10%, ± 15% and ± 20% for follow-up scans in 11 patients respectively. Mean size (craniocaudal dimension in mm) and SUVmax of primary tumor was 56 ±27 mm and 13.4 ± 4.7. Based on 18FDG PET/CT findings, patients were categorized into N0 (10/34), N1 (09/34), N2 (11/34) and N3 (04/34) while 11/32 had stage IV disease. No significant difference was seen in AC and SCC groups. CT found stage IV disease in 3/34 (09%) while PET/CT found in 11/34 (32%; p value: 0.019) cases. PET/CT showed concordance with CT in 41% while discordance (all with upstaging) seen in 59%. On follow-up PET/CT, complete metabolic response was seen in 5/11 (45%) and partial metabolic response was noted in 6/11 (55% - p value non-significant) patients. Median %∆SUVmax over primary lesions was 49.84% (-32.69 -100%) while over nodal sites it was 41.18% (-82.60 -100%).
Conclusion:
We conclude that 18FDG PET/CT was found a sensitive tool in initial staging of EC. Compared with CT, it had higher diagnostic accuracy for distant nodal and extra-nodal metastasis. %∆SUVmax between baseline and post-nCR studies acquired with standardized protocol had changed management in more than half of our patients. For response evaluation in EC more studies with standardized 18FDG PET/CT imaging protocols are warranted.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors.

- Record: found
- Abstract: found
- Article: found
The effect of SUV discretization in quantitative FDG-PET Radiomics: the need for standardized methodology in tumor texture analysis
- Record: found
- Abstract: found
- Article: not found