- Record: found
- Abstract: found
- Article: found
Remdesivir: the first FDA-approved anti-COVID-19 Treatment for Young Children
Read this article at
Abstract
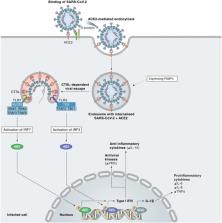
Following the emergence of the SARS-CoV-2 pandemic, finding efficient forms of treatment is seen as a priority for both adults and children. On April 25, 2022, remdesivir has become the first United States Food and Drug Administration (FDA) approved COVID-19 treatment for young children, specifically ≥28-days-old children, weighing ≥3 kilograms, who are either hospitalized or non-hospitalized, showing a high risk for progression to severe COVID-19 (prone to hospitalization or death). This new approval, which expands its already FDA-approved use in adults to young children, is supported by the CARAVAN study (a phase 2/3 single-arm, open-label study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734™) in participants, from birth to < 18 years of age, with COVID-19). This study is in progress, with an estimated primary completion in February 2023. While positive effects of remdesivir have been ascertained through various studies, controversy has surrounded remdesivir since its initial FDA approval in 2020 due to the contradictory results obtained by various studies. However, many case reports state its positive effects on the outcome of the patients, encouraging an optimistic vision for the future.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: found
Remdesivir for the Treatment of Covid-19 — Final Report
- Record: found
- Abstract: found
- Article: not found
Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis

- Record: found
- Abstract: found
- Article: found