- Record: found
- Abstract: found
- Article: not found
Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis
Read this article at
Abstract
Background
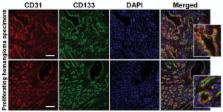
Infantile hemangioma (IH) is the most common tumor of infancy. The first-line therapy for IH is propranolol, a non-selective β-adrenergic receptor antagonist. However, mechanisms for the therapeutic effect of propranolol and regrowth of IH following cessation of treatment in some cases are not clear. We have recently shown that IH arises from multipotent stem cells. Whether IH stem cells are responsive to propranolol and are selectively targeted is unknown, and is the focus of this study.
Methods
IH stem cells were exposed to propranolol and assayed for cellular and molecular alterations. We used endothelial cells (ECs) as controls and bone marrow-mesenchymal progenitor cells (bm-MPCs) as normal stem/progenitor counterparts to determine selectivity.
Results
Our results show that propranolol significantly reduced IH stem cell growth but failed to induce caspase-3 activation. Normal bm-MPCs and mature ECs showed maintained or increased caspase-3 activation and significantly reduced cyclin-D1 levels. We further show that IH stem cells may escape apoptosis by inducing anti-apoptotic pathways.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics.
- Record: found
- Abstract: found
- Article: not found
Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells.
- Record: found
- Abstract: found
- Article: not found
