- Record: found
- Abstract: found
- Article: not found
Selective targeting of neuroblastoma tumour-initiating cells by compounds identified in stem cell-based small molecule screens
Read this article at
Abstract
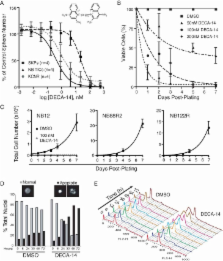
Neuroblastoma (NB) is the most deadly extra-cranial solid tumour in children necessitating an urgent need for effective and less toxic treatments. One reason for the lack of efficacious treatments may be the inability of existing drugs to target the tumour-initiating or cancer stem cell population responsible for sustaining tumour growth, metastases and relapse. Here, we describe a strategy to identify compounds that selectively target patient-derived cancer stem cell-like tumour-initiating cells (TICs) while sparing normal paediatric stem cells (skin-derived precursors, SKPs) and characterize two therapeutic candidates. DECA-14 and rapamycin were identified as NB TIC-selective agents. Both compounds induced TIC death at nanomolar concentrations in vitro, significantly reduced NB xenograft tumour weight in vivo, and dramatically decreased self-renewal or tumour-initiation capacity in treated tumours. These results demonstrate that differential drug sensitivities between TICs and normal paediatric stem cells can be exploited to identify novel, patient-specific and potentially less toxic therapies.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer.
- Record: found
- Abstract: found
- Article: not found
Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production.
- Record: found
- Abstract: found
- Article: not found
