- Record: found
- Abstract: found
- Article: not found
A validated UHPLC-MS/MS method for simultaneous quantification of some repurposed COVID-19 drugs in rat plasma: Application to a pharmacokinetic study

Read this article at
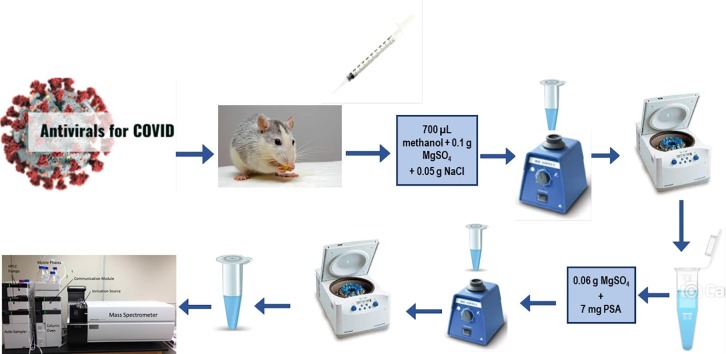
Graphical abstract
Abstract
Since the emergence of Corona virus disease (COVID-19) in 2019, a number of medications have been developed and tried to combat the pandemic. In the present study, we develop a LC-MS/MS approach to detect and quantify certain COVID-19 candidate drugs in rat plasma, including Hydroxychloroquine, Favipiravir, Oseltamivir, and Remdesivir. The analytes were separated using Ultra High-Pressure Liquid Chromatography (UHPLC) over a 13-minute run on a C 18 column. The extraction solvent for the (QuEChERS) quick, easy, cheap, effective, rugged and safe method was methanol, while the clean-up phase was primary secondary amine (PSA). Satisfactory recoveries were achieved for all compounds ranging from 82.39 to 105.87 %, with standard deviations smaller than 15.7. In terms of precision, accuracy, linearity, matrix effect, and stability, the method was validated according to US FDA criteria. The Limit of Detection (LOD) was determined to be between 0.11 and 10 ppb. The approach was further developed for a modest pharmacokinetic research in laboratory rats, and thus can be suitable for therapeutic drug monitoring in clinical cases under the same treatment.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Ion suppression; a critical review on causes, evaluation, prevention and applications.
- Record: found
- Abstract: found
- Article: not found