- Record: found
- Abstract: found
- Article: found
Dengue fatal cases present virus-specific HMGB1 response in peripheral organs
Read this article at
Abstract
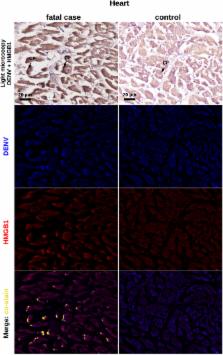
Dengue is an important infectious disease that presents high incidence and yields a relevant number of fatal cases (about 20,000) every year worldwide. Despite its epidemiological relevance, there are many knowledge gaps concerning dengue pathogenesis, especially with regards to the circumstances that drive a mild clinical course to a severe disease. In this work, we investigated the participation of high mobility group box 1 (HMGB1), an important modulator of inflammation, in dengue fatal cases. Histopathological and ultrastructural analyses revealed that liver, lung and heart post-mortem samples were marked by tissue abnormalities, such as necrosis and apoptotic cell death. These observations go in line with an HMGB1-mediated response and raised concerns regarding the participation of this cytokine in promoting/perpetuating inflammation in severe dengue. Further experiments of immunohistochemistry (IHC) showed increased expression of cytoplasmic HMGB1 in dengue-extracted tissues when compared to non-dengue controls. Co-staining of DENV RNA and HMGB1 in the host cell cytoplasm, as found by in situ hybridization and IHC, confirmed the virus specific induction of the HMGB1-mediated response in these peripheral tissues. This report brings the first in-situ evidence of the participation of HMGB1 in severe dengue and highlights novel considerations in the development of dengue immunopathogenesis.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
The extracellular release of HMGB1 during apoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
HMGB1 is an endogenous immune adjuvant released by necrotic cells.

- Record: found
- Abstract: found
- Article: found