- Record: found
- Abstract: found
- Article: found
Evaluation of Epic® label-free technology to quantify functional recombinant hemagglutinin
Read this article at
Abstract
Background
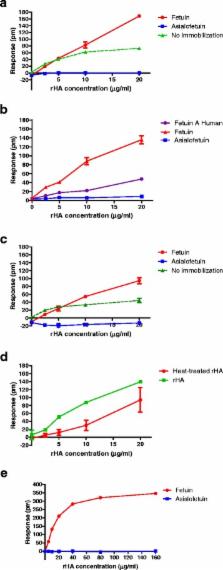
Alternative methods are being sought to measure the potency of influenza vaccines. Label-free technologies that do not require the use of hemagglutinin (HA)-specific antisera are particularly attractive as the preparation of antiserum delays availability of potency reagents. The objective of these experiments was to evaluate the use of a Corning Epic® label-free method to quantify functional influenza hemagglutinin in rHA preparations. The method was optimized to quantify recombinant HA (rHA) of B/Brisbane/60/2008 (B/BR/08). Fetuin was immobilized onto plates and the change in wavelength of refracted light measured using an Enspire (Perkin Elmer) instrument.
Results
The change in wavelength measured in response to addition of rHA of B/BR/08 was proportional to its concentration and was optimal in the presence of native rHA conformations. However, the assay was strain-dependent and did not correlate with HAU measured using turkey red blood cells.
Conclusions
The Corning Epic® label-free method is suitable for quantifying the native forms of rHA for B/BR/08 and A/Brisbane/59/2007 (H1N1) and A/Hangxhou/3/2013 (H7N9). This method is a useful tool for research purposes but further investigation is needed to identify suitable glycoproteins to use as ligands that allow quantification of HAs from a broader range of virus strains.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Label-free screening of bio-molecular interactions.
- Record: found
- Abstract: found
- Article: not found
Label-free cell-based assays with optical biosensors in drug discovery.

- Record: found
- Abstract: found
- Article: found