- Record: found
- Abstract: found
- Article: found
A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies
Read this article at
Abstract
Purpose
Anti-KIR monoclonal antibodies (mAbs) can enhance the antitumor responses of natural killer (NK) cells. We evaluated the safety of the anti-KIR2D mAb lirilumab in patients with various cancers.
Experimental design
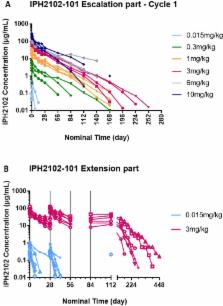
Thirty-seven patients with hematological malignancies ( n = 22) or solid tumors ( n = 15) were included in the study. Dose escalation (0.015 to 10 mg/kg) was conducted following a 3 + 3 design. Patients were scheduled to receive four cycles of treatment. In a second (extension) phase 17 patients were treated at 0.015 ( n = 9) or 3 mg/kg ( n = 8).
Results
No dose-limiting toxicity was recorded. The most frequent lirilumab-related adverse events were pruritus (19%), asthenia (16%), fatigue (14%), infusion-related reaction (14%), and headache (11%), mostly mild or moderate. Pharmacokinetics was dose-dependent and linear, with minimal accumulation resulting from the 4-weekly repeated administrations. Full KIR occupancy (>95%) was achieved with all dosages, and the duration of occupancy was dose-related. No significant changes were observed in the number or distribution of lymphocyte subpopulations, nor was any reduction in the distribution of KIR2D-positive NK cells.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
NK cells and cancer: you can teach innate cells new tricks.
- Record: found
- Abstract: found
- Article: not found
Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia.
- Record: found
- Abstract: found
- Article: not found
