- Record: found
- Abstract: found
- Article: found
Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells
Read this article at
Abstract
Introduction
A loss of p53 function resulting from mutation is prevalent in human cancers. Thus, restoration of p53 function to mutant p53 using small compounds has been extensively studied for cancer therapy. We previously reported that PRIMA-1 (for 'p53 reactivation and induction of massive apoptosis') restored the transcriptional activity of p53 target genes in breast cancer cells with a p53 mutation. By using functional proteomics approach, we sought to identify molecular targets that are involved in the restoration of normal function to mutant p53.
Methods
PRIMA-1 treated cell lysates were subjected to immunoprecipitation with DO-1 primary antibody against p53 protein, and proteins bound to p53 were separated on a denaturing gel. Bands expressed differentially between control and PRIMA-1-treated cells were then identified by matrix-assisted laser desorption ionization-time-of-flight spectrometry. Protein expression in whole cell lysates and nuclear extracts were confirmed by Western blotting. The effect of combined treatment of PRIMA-1 and adriamycin in breast cancer cells was determined with a cytotoxicity assay in vitro.
Results
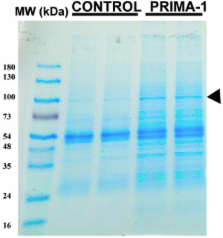
PRIMA-1 treated cells distinctly expressed a protein band of 90 kDa that was identified as heat shock protein 90 (Hsp90) by the analysis of the 90 kDa band tryptic digest. Immunoblotting with isoform-specific antibodies against Hsp90 identified this band as the α isoform of Hsp90 (Hsp90α). Co-immunoprecipitation with anti-Hsp90α antibody followed by immunoblotting with DO-1 confirmed that p53 and Hsp90α were interacting proteins. PRIMA-1 treatment also resulted in the translocation of Hsp90α to the nucleus by 8 hours. Treatment of cells with PRIMA-1 alone or in combination with adriamycin, a DNA-targeted agent, resulted in increased sensitivity of tumor cells.
Conclusion
The studies demonstrate that PRIMA-1 restores the p53-Hsp90α interaction, enhances the translocation of the p53-Hsp90α complex and reactivates p53 transcriptional activity. Our preliminary evidence also suggests that PRIMA-1 could be considered in combination therapy with DNA-targeted agents for the treatment of breast cancer, especially for tumors with aberrant p53 function.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients.
- Record: found
- Abstract: found
- Article: not found
An information-intensive approach to the molecular pharmacology of cancer.
- Record: found
- Abstract: found
- Article: not found