- Record: found
- Abstract: found
- Article: found
PI3K-targeting strategy using alpelisib to enhance the antitumor effect of paclitaxel in human gastric cancer
Read this article at
Abstract
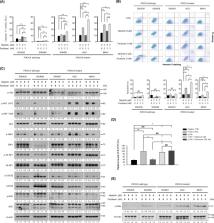
PIK3CA mutations are frequently observed in various human cancers including gastric cancer (GC). This study was conducted to investigate the anti-tumor effects of alpelisib, a PI3K p110α-specific inhibitor, using preclinical models of GC. In addition, the combined effects of alpelisib and paclitaxel on GC were evaluated. Among the SNU1, SNU16, SNU484, SNU601, SNU638, SNU668, AGS, and MKN1 GC cells, three PIK3CA-mutant cells were predominantly sensitive to alpelisib. Alpelisib monotherapy decreased AKT and S6K1 phosphorylation and induced G 0/G 1 phase arrest regardless of PIK3CA mutational status. The alpelisib and paclitaxel combination demonstrated synergistic anti-proliferative effects, preferentially on PIK3CA-mutant cells, resulting in increased DNA damage response and apoptosis. In addition, alpelisib and paclitaxel combination potentiated anti-migratory activity in PIK3CA-mutant cells. Alpelisib partially reversed epithelial–mesenchymal transition markers in PIK3CA-mutant cells. In a xenograft model of MKN1 cells, the alpelisib and paclitaxel combination significantly enhanced anti-tumor activity by decreasing Ki-67 expression and increasing apoptosis. Moreover, this combination tended to prolong the survival of tumor-bearing mice. Our data suggest promising anti-tumor efficacy of alpelisib alone or in combination with paclitaxel in PIK3CA-mutant GC cells.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Oncogenic mutations of PIK3CA in human cancers.
- Record: found
- Abstract: found
- Article: not found
PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer.
- Record: found
- Abstract: found
- Article: not found