- Record: found
- Abstract: found
- Article: found
Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Read this article at
Abstract
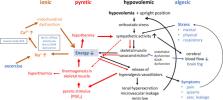
Chronic Fatigue Syndrome or Myalgic Encephaloymelitis (ME/CFS) is a frequent debilitating disease with an enigmatic etiology. The finding of autoantibodies against ß2-adrenergic receptors (ß2AdR) prompted us to hypothesize that ß2AdR dysfunction is of critical importance in the pathophysiology of ME/CFS. Our hypothesis published previously considers ME/CFS as a disease caused by a dysfunctional autonomic nervous system (ANS) system: sympathetic overactivity in the presence of vascular dysregulation by ß2AdR dysfunction causes predominance of vasoconstrictor influences in brain and skeletal muscles, which in the latter is opposed by the metabolically stimulated release of endogenous vasodilators (functional sympatholysis). An enigmatic bioenergetic disturbance in skeletal muscle strongly contributes to this release. Excessive generation of these vasodilators with algesic properties and spillover into the systemic circulation could explain hypovolemia, suppression of renin (paradoxon) and the enigmatic symptoms. In this hypothesis paper the mechanisms underlying the energetic disturbance in muscles will be explained and merged with the first hypothesis. The key information is that ß2AdR also stimulates the Na +/K +-ATPase in skeletal muscles. Appropriate muscular perfusion as well as function of the Na +/K +-ATPase determine muscle fatigability. We presume that dysfunction of the ß2AdR also leads to an insufficient stimulation of the Na +/K +-ATPase causing sodium overload which reverses the transport direction of the sodium-calcium exchanger (NCX) to import calcium instead of exporting it as is also known from the ischemia–reperfusion paradigm. The ensuing calcium overload affects the mitochondria, cytoplasmatic metabolism and the endothelium which further worsens the energetic situation (vicious circle) to explain postexertional malaise, exercise intolerance and chronification. Reduced Na +/K +-ATPase activity is not the only cause for cellular sodium loading. In poor energetic situations increased proton production raises intracellular sodium via sodium-proton-exchanger subtype-1 (NHE1), the most important proton-extruder in skeletal muscle. Finally, sodium overload is due to diminished sodium outward transport and enhanced cellular sodium loading. As soon as this disturbance would have occurred in a severe manner the threshold for re-induction would be strongly lowered, mainly due to an upregulated NHE1, so that it could repeat at low levels of exercise, even by activities of everyday life, re-inducing mitochondrial, metabolic and vascular dysfunction to perpetuate the disease.
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
COVID-19 is, in the end, an endothelial disease
- Record: found
- Abstract: found
- Article: not found
NCLX is an essential component of mitochondrial Na+/Ca2+ exchange.
- Record: found
- Abstract: not found
- Article: not found