- Record: found
- Abstract: found
- Article: found
Dipeptidyl Peptidase‐4 Inhibition Potentiates Stimulated Growth Hormone Secretion and Vasodilation in Women
Read this article at
Abstract
Background
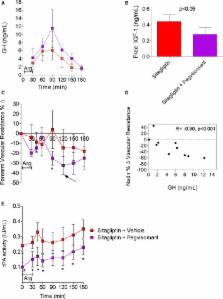
Diminished growth hormone ( GH) is associated with impaired endothelial function and fibrinolysis. GH‐releasing hormone is the primary stimulus for GH secretion and a substrate of dipeptidyl peptidase‐4. We tested the hypothesis that dipeptidyl peptidase‐4 inhibition with sitagliptin increases stimulated GH secretion, vasodilation, and tissue plasminogen activator ( tPA) activity.
Methods and Results
Healthy adults participated in a 2‐part double‐blind, randomized, placebo‐controlled, crossover study. First, 39 patients (29 women) received sitagliptin or placebo on each of 2 days separated by a washout. One hour after study drug, blood was sampled and then arginine (30 g IV) was given to stimulate GH. Vasodilation was assessed by plethysmography and blood sampled for 150 minutes. Following a washout, 19 of the original 29 women received sitagliptin alone versus sitagliptin plus antagonist to delineate GH receptor ( GHR)– (n=5), nitric oxide– (n=7), or glucagon‐like peptide‐1 receptor– (n=7) dependent effects. Sitagliptin enhanced stimulated GH secretion ( P<0.01 versus placebo, for 30 minutes) and free insulin–like growth factor‐1 ( P<0.001 versus placebo, after adjustment for baseline) in women. Vasodilation and tPA increased in all patients, but sitagliptin enhanced vasodilation ( P=0.01 versus placebo) and increased tPA ( P<0.001) in women only. GHR blockade decreased free insulin–like growth factor‐1 ( P=0.04 versus sitagliptin alone) and increased stimulated GH ( P<0.01), but decreased vascular resistance ( P=0.01) such that nadir vascular resistance correlated inversely with GH ( r s=−0.90, P<0.001). GHR blockade suppressed tPA. Neither nitric oxide nor glucagon‐like peptide‐1 receptor blockade affected vasodilation or tPA.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways.
- Record: found
- Abstract: found
- Article: not found
Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study.
- Record: found
- Abstract: found
- Article: not found