- Record: found
- Abstract: found
- Article: found
Fabrication of Asymmetric Supercapacitors (AC@GQDs//AC) with High Electrochemical Performance Utilizing Activated Carbon and Graphene Quantum Dots

Read this article at
Abstract
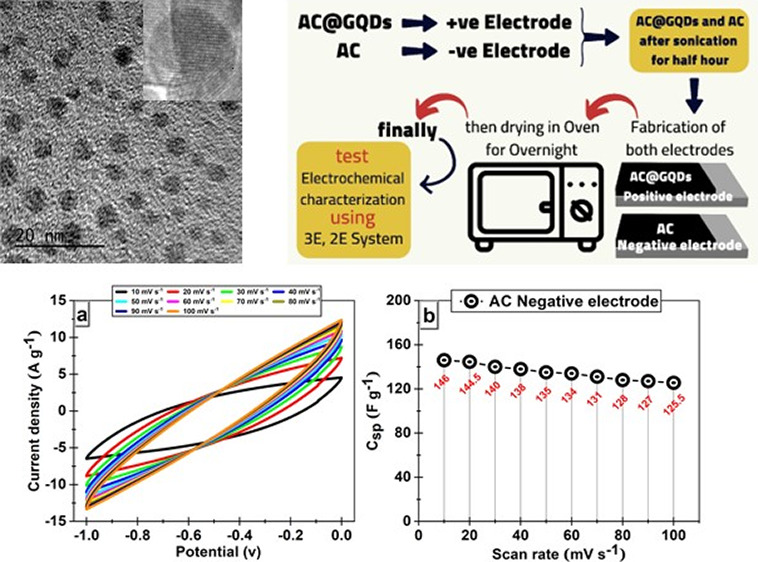
Sugar cane bagasse stands as a prevalent and abundant form of solid agricultural waste, making it a prime candidate for innovative utilization. Harnessing its potential, we embarked on a groundbreaking endeavor to evaluate the sustainability of a molasses-based hydrothermal process to produce graphene quantum dots (GQDs). This pioneering initiative promises remarkable environmental benefits and holds immense economic potential. Embedding crystalline GQDs in activated carbon (AC) boost electrochemical efficiency by enhancing charge-transfer and ion migration kinetics. Optical, structural, and morphological evaluations were used to confirm the formation of GQDs. Transmission electron microscopy (TEM) investigation showed the size, shape, and fact that GQDs were monodispersed, and X-ray diffraction and Fourier transform infrared determined the structure of GQDs. The electrodes with negative (AC) and positive (AC@GQDs) polarity demonstrate a considerable specific capacitance of 220 and 265 F g –1, respectively, when measured at 0.5 A g –1. Additionally, these electrodes exhibit high-rate capabilities of 165 and 230 F g –1 when measured at 5 A g –1, as determined by galvanostatic charge–discharge techniques. The supercapacitor device comprising asymmetric AC//AC@GQDs exhibits a specific capacitance of 118 F g –1. Furthermore, the asymmetric device exhibits exceptional cycling behavior, with an impressive 92% capacitance retention even after undergoing 10,000 cycles. This remarkable performance underscores the immense potential of both the negative and positive electrodes for real-world supercapacitor applications. Such findings pave the way for promising advancements in the field and offer exciting prospects for practical utilization.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications
- Record: found
- Abstract: found
- Article: not found
Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities
- Record: found
- Abstract: not found
- Article: not found
