- Record: found
- Abstract: found
- Article: not found
Cachexia and protein-energy wasting in children with chronic kidney disease
Read this article at
Abstract
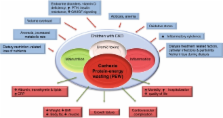
Children with chronic kidney disease (CKD) are at risk for “cachexia” or “protein-energy wasting” (PEW). These terms describe a pathophysiologic process resulting in the loss of muscle, with or without loss of fat, and involving maladaptive responses, including anorexia and elevated metabolic rate. PEW has been defined specifically in relation to CKD. We review the diagnostic criteria for cachexia and PEW in CKD and consider the limitations and applicability of these criteria to children with CKD. In addition, we present an overview of the manifestations and mechanisms of cachexia and PEW. A host of pathogenetic factors are considered, including systemic inflammation, endocrine perturbations, and abnormal neuropeptide signaling, as well as poor nutritional intake. Mortality risk, which is 100- to 200-fold higher in patients with end-stage renal disease than in the general population, is strongly correlated with the components of cachexia/PEW. Further research into the causes and consequences of wasting and growth retardation is needed in order to improve the survival and quality of life for children with CKD.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients.
- Record: found
- Abstract: found
- Article: not found
Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences.
- Record: found
- Abstract: found
- Article: not found
