- Record: found
- Abstract: found
- Article: found
Outcomes in Localized Prostate Cancer: National Prostate Cancer Register of Sweden Follow-up Study
Read this article at
Abstract
Background
Treatment for localized prostate cancer remains controversial. To our knowledge, there are no outcome studies from contemporary population-based cohorts that include data on stage, Gleason score, and serum levels of prostate-specific antigen (PSA).
Methods
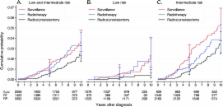
In the National Prostate Cancer Register of Sweden Follow-up Study, a nationwide cohort, we identified 6849 patients aged 70 years or younger. Inclusion criteria were diagnosis with local clinical stage T1–2 prostate cancer from January 1, 1997, through December 31, 2002, a Gleason score of 7 or less, a serum PSA level of less than 20 ng/mL, and treatment with surveillance (including active surveillance and watchful waiting, n = 2021) or curative intent (including radical prostatectomy, n = 3399, and radiation therapy, n = 1429). Among the 6849 patients, 2686 had low-risk prostate cancer (ie, clinical stage T1, Gleason score 2-6, and serum PSA level of <10 ng/mL). The study cohort was linked to the Cause of Death Register, and cumulative incidence of death from prostate cancer and competing causes was calculated.
Results
For the combination of low- and intermediate-risk prostate cancers, calculated cumulative 10-year prostate cancer–specific mortality was 3.6% (95% confidence interval [CI] = 2.7% to 4.8%) in the surveillance group and 2.7% (95% CI = 2.1% to 3.45) in the curative intent group. For those with low-risk disease, the corresponding values were 2.4% (95% CI = 1.2% to 4.1%) among the 1085 patients in the surveillance group and 0.7% (95% CI = 0.3% to 1.4%) among the 1601 patients in the curative intent group. The 10-year risk of dying from competing causes was 19.2% (95% CI = 17.2% to 21.3%) in the surveillance group and 10.2% (95% CI = 9.0% to 11.4%) in the curative intent group.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Estimation of failure probabilities in the presence of competing risks: new representations of old estimators.
- Record: found
- Abstract: found
- Article: not found
Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer.
- Record: found
- Abstract: found
- Article: not found