- Record: found
- Abstract: found
- Article: found
Treatment with Riluzole Restores Normal Control of Soleus and Extensor Digitorum Longus Muscles during Locomotion in Adult Rats after Sciatic Nerve Crush at Birth
Read this article at
Abstract
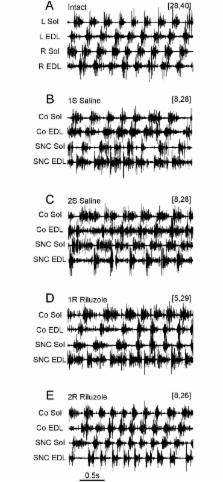
The effects of sciatic nerve crush (SNC) and treatment with Riluzole on muscle activity during unrestrained locomotion were identified in an animal model by analysis of the EMG activity recorded from soleus (Sol) and extensor digitorum longus (EDL) muscles of both hindlimbs; in intact rats (IN) and in groups of rats treated for 14 days with saline (S) or Riluzole (R) after right limb nerve crush at the 1 st (1S and 1R) or 2 nd (2S and 2R) day after birth. Changes in the locomotor pattern of EMG activity were correlated with the numbers of survived motor units (MUs) identified in investigated muscles. S rats with 2–8 and 10–28 MUs that survived in Sol and EDL muscles respectively showed increases in the duration and duty factor of muscle EMG activity and a loss of correlation between the duty factors of muscle activity, and abnormal flexor-extensor co-activation 3 months after SNC. R rats with 5, 6 (Sol) and 15–29 MUs (EDL) developed almost normal EMG activity of both Sol and control EDL muscles, whereas EDL muscles with SNC showed a lack of recovery. R rats with 8 (Sol) and 23–33 (EDL) MUs developed almost normal EMG activities of all four muscles. A subgroup of S rats with a lack of recovery and R rats with almost complete recovery that had similar number of MUs (8 and 24–28 vs 8 and 23–26), showed that the number of MUs was not the only determinant of treatment effectiveness. The results demonstrated that rats with SNC failed to develop normal muscle activity due to malfunction of neuronal circuits attenuating EDL muscle activity during the stance phase, whereas treatment with Riluzole enabled almost normal EMG activity of Sol and EDL muscles during locomotor movement.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Organization of mammalian locomotor rhythm and pattern generation.
- Record: found
- Abstract: found
- Article: not found