- Record: found
- Abstract: found
- Article: found
MCC1019, a selective inhibitor of the Polo-box domain of Polo-like kinase 1 as novel, potent anticancer candidate
Read this article at
Abstract
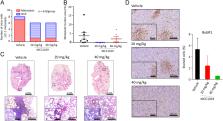
Polo-like kinase (PLK1) has been identified as a potential target for cancer treatment. Although a number of small molecules have been investigated as PLK1 inhibitors, many of which showed limited selectivity. PLK1 harbors a regulatory domain, the Polo box domain (PBD), which has a key regulatory function for kinase activity and substrate recognition. We report on 3-bromomethyl-benzofuran-2-carboxylic acid ethyl ester (designated: MCC1019) as selective PLK1 inhibitor targeting PLK1 PBD. Cytotoxicity and fluorescence polarization-based screening were applied to a library of 1162 drug-like compounds to identify potential inhibitors of PLK1 PBD. The activity of compound MC1019 against the PLK1 PBD was confirmed using fluorescence polarization and microscale thermophoresis. This compound exerted specificity towards PLK1 over PLK2 and PLK3. MCC1019 showed cytotoxic activity in a panel of different cancer cell lines. Mechanistic investigations in A549 lung adenocarcinoma cells revealed that MCC1019 induced cell growth inhibition through inactivation of AKT signaling pathway, it also induced prolonged mitotic arrest—a phenomenon known as mitotic catastrophe, which is followed by immediate cell death via apoptosis and necroptosis. MCC1019 significantly inhibited tumor growth in vivo in a murine lung cancer model without affecting body weight or vital organ size, and reduced the growth of metastatic lesions in the lung. We propose MCC1019 as promising anti-cancer drug candidate.
Graphical abstract
MCC1019 is a novel anticancer candidate that selectively targets PLK1. It mediates G2/M cell cycle arrest and cell death through induction of apoptosis and necroptosis. Inhibition of PLK1 downstream effectors like spindle assembly check points and cell growth pathway was well characterized. In vivo models revealed inhibition of tumor growth and metastasis.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Akt, FoxO and regulation of apoptosis.
- Record: found
- Abstract: found
- Article: not found
FoxO transcription factors; Regulation by AKT and 14-3-3 proteins.
- Record: found
- Abstract: found
- Article: not found
