- Record: found
- Abstract: found
- Article: found
Neuraxial Dexmedetomidine: Wonder Drug or Simply Harmful
editorial
30 March 2015
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
In current anesthesia practice, neuraxial anesthesia is a major method among all anesthesia
modalities. The most frequently performed neuraxial blocks are consecutively subarachnoid,
epidural, and caudal blocks. Major indications are intraoperative anesthesia and analgesia,
postoperative analgesia, analgesia for vaginal delivery, and management of chronic
pain. For these purpose, local anesthetics (LA) are widely used alone or in combination
with adjuvants. Adjuvants are mixed with LA to shorten the onset of action, increase
the quality of block, increase the duration of anesthesia and analgesia, and to decrease
the dose of LA. Benzodiazepines (e.g. midazolam), opioids (e.g. morphine, fentanyl,
and sufentanil), α-adrenergic agonists (e.g. epinephrine or phenylephrine), ketamine,
and ∝2-adrenergic receptor agonists (e.g. clonidine or dexmedetomidine [DEX]) are
adjuvants of common use. Mechanisms of action are also different. Out of these, ∝2-adrenergic
receptor agonists are relatively newer and their uses are increasing. DEX, a dextrorotatory
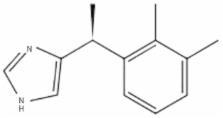
S-enantiomer of medetomidine, is an ∝2-adrenergic receptor agonist with the chemical
structure being (S)-4-[1-(2, 3-dimethylphenyl) ethyl]-3H-Imidazole (Figure 1).
Figure 1.
Chemical Structure of Dexmedetomidine
Like clonidine, DEX also acts on both α1 and α2 receptors. The α1 to α2 receptors
binding ratio of 1:1620 makes it a highly selective α2 agonist in comparison to clonidine.
Hence, the advantage of DEX over clonidine is decreased incidence of adverse effects
due to involvement of α1 receptors (1). In 1999, the Food and Drug Administration
(FDA) approved DEX use for short-term sedation and analgesia (< 24 hours) in the intensive
care units (ICU). In perioperative period, intravenous infusion of DEX acts as an
anxiolytic and analgesic that blunts sympathetic response to laryngoscopy and intubation
(2-4), decreases sympathetic response and emergent reaction on extubation with no
delay in recovery or prolonged sedation (5, 6), and decreases the need for anesthetic
agents (intravenous and inhalational), cardiovascular stabilization, neuroprotection,
renoprotection, and no or minimal respiratory depression, reduced postoperative shivering
(7). In critically ill patients, DEX is a useful sedative agent with analgesic properties,
hemodynamic stability, and ability to recover respiratory function in mechanically
ventilated patients facilitating early weaning. Other claimed advantages are reduced
ICU stay, decreased duration of ventilation, and reduced agitation (8, 9). Although
FDA has not approved DEX as an adjuvant in neuraxial blocks, it is widely used and
is still in use in anesthesia practice as an adjuvant in regional anesthesia for both
epidural and intrathecal modalities. Most of these trials, which had used DEX in intrathecal
and epidural block, were conducted with or without prior approval of Institutional
Ethics Committees. FDA and Drug Controller General of India (DCGI) do not approve
"off-label use" of DEX as intrathecal or epidural adjuvant. Although outcome of most
of these studies is favorable for its use as an adjuvant for neuraxial anesthesia,
approval by FDA and DCGI is desirable for its uncontroversial use in anesthesia practice.
Adding an adjuvant to the LA in subarachnoid or epidural space needs complete information
regarding its specific gravity, pH, and compatibility as well as stability of the
LA and adjuvant mixture; at present, no study has done or at least reported such information.
In neuraxial anesthesia, DEX mediates its analgesic effects via spinal α2 receptors
by depressing the release of C-fiber neurotransmitters and by hyperpolarization of
postsynaptic dorsal horn neurons (10). Binding of α2 adrenoceptor agonists to motor
neurons in the dorsal horn explains the motor effect of DEX. When used as an adjuvant
to LA for neuraxial block, DEX leads to (10-14):
Reduced onset time of sensory and motor block,
increased duration of sensory block,
delayed motor regression,
prolonged postoperative analgesia and reduced total dose of analgesic,
Delayed need of first rescue analgesic,
Decreased postoperative shivering.
Effects are usually dose dependent. Until now, there is no specific recommended dose
of DEX for this purpose. Dose can be varied from 3 to 15 µg as an adjuvant to LA in
spinal anesthesia. Solanki et al. stated that in comparison to clonidine (50 µg),
equipotent dose of intrathecal DEX (5 µg) significantly prolonged postoperative analgesia
(10). When compared to other adjuvants (fentanyl, magnesium sulfate, or buprenorphine),
DEX showed prolonged postoperative analgesia with delayed and decreased need of rescue
analgesics (11-13). For caudal epidural block, 1 to 2 µg/kg of DEX along with bupivacaine
led to prolonged analgesia without significant side effects (14, 15). Moreover, use
of epidural DEX significantly decreases the anesthetic requirements, prevents awareness
during anesthesia, and improves intraoperative oxygenation and postoperative analgesia
(16, 17). DEX along with LA for epidural analgesia during labor pains shows good maternal
satisfaction without deleterious effect on uteroplacental circulation and newborns
outcome (18). In a review and meta-analysis of perineural DEX, Abdallah et al. (19)
showed an association between intrathecal or epidural DEX, as adjuvant to LA, and
onset and duration of sensory and motor blockade; moreover, the time to first analgesic
request was prolonged. They also mentioned that these results might be due to publication
bias because of the source studies may or may not reflect less stringent Institutional
Review Board and/or editorial board policies. DEX has also been used as an adjuvant
in peripheral nerve blocks and has shown to prolong the sensory and motor block duration
(20). The most common reported adverse effects are bradycardia and hypotension. Bradycardia
due to DEX is resistant to atropine and higher doses are needed; although rare, even
cardiac arrest might occur. Reported fatal complications of DEX were mainly related
to its intravenous use as infusion in the elderly and in patients with cardiac disease
(21, 22). Hypotension is due to decrease in central sympathetic outflow. When a large
dose of DEX is used, it is preceded by hypertensive episode due to stimulation of
α-2B receptors. Klinger et al. (23) in a retrospective analysis of 15656 patients
concluded that there was no significant difference in the overall incidence of intraoperative
hypotension (5.3% in DEX group, 6% in control group) or bradycardia (0.4% in both
groups); however phenylephrine or atropine were more required in DEX group (23% vs.
15%; P < 0.0001). DEX should not be used as a sole anesthetic for neuraxial anesthesia.
Konakci et al. (24), in their study on rabbits, observed that when epidural DEX was
administered without LA, it would induce neurotoxicity (evidence of demyelination
of the oligodendrocytes in the white matter in DEX group) in doses as high as 6.1
µg/kg. None of the human randomized studies have used DEX doses > 0.2 µg/kg for spinal
adjuvant and > 1 µg/kg for epidural adjuvant. In addition, intrathecal DEX has shown
a neuroprotective effect similar to methylprednisolone (25, 26), and no long-term
and irreversible harmful effect of neuraxial DEX has been reported yet.
In conclusion, DEX is a good LA adjuvant that can hasten the onset and prolong the
duration of sensory and motor blockade when used in intrathecal or epidural block
and appears to be safe; however, there are insufficient safety data to support the
use of neuraxial DEX in the clinical setting.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
The effects of increasing plasma concentrations of dexmedetomidine in humans.
Thomas Ebert, Judith Hall, Jill A. Barney … (2000)
- Record: found
- Abstract: found
- Article: not found
Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes.
Jordan D. Ward, B C Bloor, J A Belleville … (1992)
- Record: found
- Abstract: found
- Article: not found
Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis.
F Abdallah, R Brull (2013)