- Record: found
- Abstract: found
- Article: found
OsABCG9 Is an Important ABC Transporter of Cuticular Wax Deposition in Rice
Read this article at
Abstract
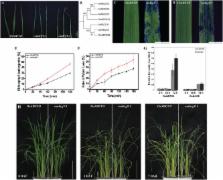
The importance of the cuticular layer in regulating a plant’s water status and providing protection from environmental challenges has been recognized for a long time. The cuticular layer in plants restricts non-stomatal water loss and protects plants against damage from pathogen infection and UV radiation. Much genetic and biochemical research has been done about cutin and wax transportation in Arabidopsis thaliana, but little is known about it in rice. Here, we report that a rice ATP-binding cassette (ABC) transporter, OsABCG9, is essential for normal development during vegetative growth and could play a critical role in the transportation of epicuticular wax in rice. Rice phenotypes with mutated OsABCG9 exhibited growth retardation and sensitivity to low humidity. The total amount of cuticular wax on the leaves of the osabcg9-1 mutant diminished by 53% compared with the wild type, and wax crystals disappeared completely in osabcg9-2 mutant leaves. However, OsABCG9 does not seem to be involved in cutin transportation, even though its ortholog in Arabidopsis, AtABCG11, transports both wax and cutin. Furthermore, the osabcg9-1 mutant had increased leaf chlorophyll leaching and more severe drought susceptibility. This study provides new insights about differences between rice and A. thaliana in wax and cutin transportation associated with the ABCG family during evolution.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Sealing plant surfaces: cuticular wax formation by epidermal cells.
- Record: found
- Abstract: found
- Article: not found
Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice.
- Record: found
- Abstract: found
- Article: not found
