- Record: found
- Abstract: found
- Article: found
DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment
Read this article at
Abstract
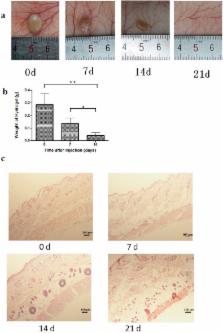
Melanoma has been a serious threat to the human health; however, effective therapeutic methods of this cancer are still limited. Combined local therapy is a crucial approach for achieving a superior anti-tumor efficacy. In this paper, a chemo-immunotherapy system of DOX, IL-2 and IFN-γ based on poly(γ-ethyl-L-glutamate)-poly(ethylene glycol)-poly(γ-ethyl-L-glutamate) (PELG-PEG-PELG) hydrogel was developed for local treatment of melanoma xenograft. The drug release process of this system exhibited a short term of burst release (the first 3 days), followed by a long-term sustained release (the following 26 days). The hydrogel degraded completely within 3 weeks without obvious inflammatory responses in the subcutaneous layer of rats, showing a good biodegradability and biocompatibility. The DOX/IL-2/IFN-γ co-loaded hydrogel also showed enhanced anti-tumor effect against B16F10 cells in vitro, through increasing the ratio of cell apoptosis and G2/S phage cycle arrest. Moreover, the combined strategy presented improved therapy efficacy against B16F10 melanoma xenograft without obvious systemic side effects in a nude mice model, which was likely related to both the enhanced tumor cell apoptosis and the increased proliferation of the CD3 +/CD4 + T-lymphocytes and CD3 +/CD8 + T-lymphocytes. Overall, the strategy of localized co-delivery of DOX/IL-2/IFN-γ using the polypeptide hydrogel provided a promising approach for efficient melanoma therapy.
Graphical abstract
Schematic illustration for the mechanism of DOX/IL-2/IFN-γ co-loaded hydrogel of PELG 7-PEG 45-PELG 7 on melanoma local treatment in nude mice: the released drugs from the hydrogel could induce the cell apoptosis as well as promote the proliferation of CD3 +/CD4 + T-lymphocytes and CD3 +/CD8 + T-lymphocytes to cause enhanced anti-tumor efficacy against melanoma xenograft in mice model.
Highlights
-
•
A chemo-immunotherapy combined system based on polypeptide hydrogel was developed for the local therapy of melanoma.
-
•
The combined strategy presented enhanced anti-tumor effect on B16F10 cells in vitro through different mechanisms.
-
•
The combined treatment showed improved efficacy against B16F10 melanoma xenograft with less systemic toxicity in vivo.
Related collections
Most cited references29
- Record: found
- Abstract: not found
- Article: not found
Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma.
- Record: found
- Abstract: found
- Article: not found
Immune therapy for cancer.

- Record: found
- Abstract: found
- Article: found
