- Record: found
- Abstract: found
- Article: found
Verification of cardiac mechanics software: benchmark problems and solutions for testing active and passive material behaviour
Read this article at
Abstract
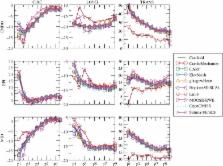
Models of cardiac mechanics are increasingly used to investigate cardiac physiology. These models are characterized by a high level of complexity, including the particular anisotropic material properties of biological tissue and the actively contracting material. A large number of independent simulation codes have been developed, but a consistent way of verifying the accuracy and replicability of simulations is lacking. To aid in the verification of current and future cardiac mechanics solvers, this study provides three benchmark problems for cardiac mechanics. These benchmark problems test the ability to accurately simulate pressure-type forces that depend on the deformed objects geometry, anisotropic and spatially varying material properties similar to those seen in the left ventricle and active contractile forces. The benchmark was solved by 11 different groups to generate consensus solutions, with typical differences in higher-resolution solutions at approximately 0.5%, and consistent results between linear, quadratic and cubic finite elements as well as different approaches to simulating incompressible materials. Online tools and solutions are made available to allow these tests to be effectively used in verification of future cardiac mechanics software.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
Verification of cardiac tissue electrophysiology simulators using an N-version benchmark.
- Record: found
- Abstract: found
- Article: not found
Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease.
- Record: found
- Abstract: found
- Article: not found