- Record: found
- Abstract: found
- Article: found
A Quantum Chemical and Statistical Study of Phenolic Schiff Bases with Antioxidant Activity against DPPH Free Radical
Read this article at
Abstract
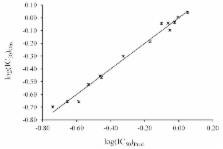
Phenolic Schiff bases are known as powerful antioxidants. To select the electronic, 2D and 3D descriptors responsible for the free radical scavenging ability of a series of 30 phenolic Schiff bases, a set of molecular descriptors were calculated by using B3P86 (Becke’s three parameter hybrid functional with Perdew 86 correlation functional) combined with 6-31 + G(d,p) basis set ( i.e., at the B3P86/6-31 + G(d,p) level of theory). The chemometric methods, simple and multiple linear regressions (SLR and MLR), principal component analysis (PCA) and hierarchical cluster analysis (HCA) were employed to reduce the dimensionality and to investigate the relationship between the calculated descriptors and the antioxidant activity. The results showed that the antioxidant activity mainly depends on the first and second bond dissociation enthalpies of phenolic hydroxyl groups, the dipole moment and the hydrophobicity descriptors. The antioxidant activity is inversely proportional to the main descriptors. The selected descriptors discriminate the Schiff bases into active and inactive antioxidants.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Structure-antioxidant activity relationships of flavonoids and phenolic acids.
- Record: found
- Abstract: not found
- Article: not found
The molecular basis of working mechanism of natural polyphenolic antioxidants
- Record: found
- Abstract: not found
- Article: not found