- Record: found
- Abstract: found
- Article: found
Mechanoresponsive musculoskeletal tissue differentiation of adipose-derived stem cells
Read this article at
Abstract
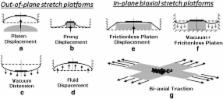
Musculoskeletal tissues are constantly under mechanical strains within their microenvironment. Yet, little is understood about the effect of in vivo mechanical milieu strains on cell development and function. Thus, this review article outlines the in vivo mechanical environment of bone, muscle, cartilage, tendon, and ligaments, and tabulates the mechanical strain and stress in these tissues during physiological condition, vigorous, and moderate activities. This review article further discusses the principles of mechanical loading platforms to create physiologically relevant mechanical milieu in vitro for musculoskeletal tissue regeneration. A special emphasis is placed on adipose-derived stem cells (ADSCs) as an emerging valuable tool for regenerative musculoskeletal tissue engineering, as they are easily isolated, expanded, and able to differentiate into any musculoskeletal tissue. Finally, it highlights the current state-of-the art in ADSCs-guided musculoskeletal tissue regeneration under mechanical loading.
Related collections
Most cited references139
- Record: found
- Abstract: found
- Article: not found
Mechanical properties and the hierarchical structure of bone.
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease.
- Record: found
- Abstract: found
- Article: not found