- Record: found
- Abstract: found
- Article: found
Trimethoprim Use prior to Pregnancy and the Risk of Congenital Malformation: A Register-Based Nationwide Cohort Study
Read this article at
Abstract
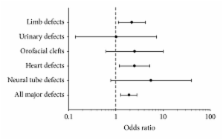
Objectives. The aim of the study was to investigate whether the use of the antifolate antibiotic trimethoprim during the 12 weeks before conception was associated with congenital malformations. Methods. We conducted a nationwide register-based cohort study including all Danish women giving birth from 1997 to 2004. All women with at least one prescription of trimethoprim dispensed during the 12 weeks before conception were identified. Results. There was a doubling of congenital malformations in offspring to women exposed to trimethoprim in the 12 weeks before conception. The adjusted odds ratio (OR) of major congenital malformation was 1.87, 95% confidence interval (CI) 1.25–2.81. There was a significant increase in major malformations of the heart (OR = 2.49; 1.18–5.26) and limbs (OR = 2.18; 1.13–4.23). Conclusions. In this study, we found an association between exposure to trimethoprim during the 12 weeks before conception and an increased risk of heart and limb defects.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention.
- Record: found
- Abstract: found
- Article: not found
Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group.
- Record: found
- Abstract: found
- Article: not found