- Record: found
- Abstract: found
- Article: found
Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model
Read this article at
Abstract
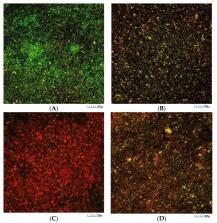
In search for natural products with antimicrobial properties for use in the prevention and treatment of periodontitis, the purpose of this investigation was to evaluate the antimicrobial activity of two omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), using an in vitro multi-species subgingival biofilm model including Streptococcus oralis, Actinomyces naeslundii, Veillonella parvula, Fusobacterium nucleatum, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans. The antimicrobial activities of EPA and DHA extracts (100 µM) and the respective controls were assessed on 72 h biofilms by their submersion onto discs for 60 s. Antimicrobial activity was evaluated by quantitative polymerase chain reaction (qPCR), confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM). ANOVA with Bonferroni correction was used to evaluate the antimicrobial activity of each of the fatty acids. Both DHA and EPA significantly reduced ( p < 0.001 in all cases) the bacterial strains used in this biofilm model. The results with CLSM were consistent with those reported with qPCR. Structural damage was evidenced by SEM in some of the observed bacteria. It was concluded that both DHA and EPA have significant antimicrobial activity against the six bacterial species included in this biofilm model.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential.
- Record: found
- Abstract: found
- Article: not found
Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology.
- Record: found
- Abstract: found
- Article: not found