- Record: found
- Abstract: found
- Article: found
Transcriptome profiling reveals superovulation with the gonadotropin-releasing hormone agonist trigger impaired embryo implantation in mice
Read this article at
Abstract
Introduction
Superovulation is a critical step in assisted reproductive technology, but the use of human chorionic gonadotropin (hCG) as a trigger for superovulation can result in ovarian hyperstimulation. Thus, the use of Gonadotropin-releasing hormone agonist (GnRHa) trigger has been increasingly adopted, although it has been associated with a higher rate of pregnancy failure compared to natural cycles. This study aimed to investigate the effect of GnRHa trigger on embryo implantation in a mouse model.
Methods
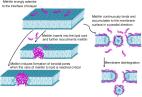
Mice in the superovulation (PG) group were administered 7.5 IU of PMSG, followed by the injection of 3.5 μg of GnRHa (Leuprorelin) 48 h later, while mice in the control group (CTR) mated naturally. We compared the number of oocytes, blastocysts, and corpus luteum between the two groups and the implantation sites after the transfer of natural blastocysts. Ovaries, uterus, and serum 2 and 4 days after mating were collected for qRT-PCR, transcriptome sequencing, and hormone assays.
Results
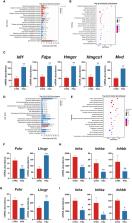
The PG group had more oocytes, blastocysts, and corpus luteum after superovulation than the CTR group. However, the mRNA expression of leukemia inhibitory factor ( Lif) and the number of implantation sites were reduced in the PG group. The ELISA assay revealed that superovulation increased ovarian estrogen secretion. The transcriptome analysis showed that superphysiological estrogen led to a response of the uterus to a high estrogen signal, resulting in abnormal endometrium and extracellular matrix remodeling and up-regulation of ion transport and inflammation-related genes.
Conclusion
Our findings suggest that a combination of PMSG and GnRHa trigger impaired embryo implantation in mice, as the excessive uterine response to superphysiological estrogen levels can lead to the change of gene expression related to endometrial remodeling, abnormal expression of uterine ion transport genes and excessive immune-related genes.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Molecular cues to implantation.
- Record: found
- Abstract: found
- Article: not found
Roadmap to embryo implantation: clues from mouse models.
- Record: found
- Abstract: found
- Article: not found