- Record: found
- Abstract: found
- Article: found
Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis
Read this article at
Abstract
Objective
Gut microbiota is a key player in dictating immunotherapy response. We aimed to explore the immunomodulatory effect of probiotic Lactobacillus gallinarum and its role in improving anti-programmed cell death protein 1 (PD1) efficacy against colorectal cancer (CRC).
Design
The effects of L. gallinarum in anti-PD1 response were assessed in syngeneic mouse models and azoxymethane/dextran sulfate sodium-induced CRC model. The change of immune landscape was identified by multicolour flow cytometry and validated by immunohistochemistry staining and in vitro functional assays. Liquid chromatography-mass spectrometry was performed to identify the functional metabolites.
Results
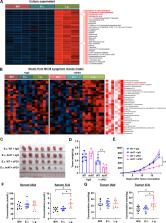
L. gallinarum significantly improved anti-PD1 efficacy in two syngeneic mouse models with different microsatellite instability (MSI) statuses (MSI-high for MC38, MSI-low for CT26). Such effect was confirmed in CRC tumourigenesis model. L. gallinarum synergised with anti-PD1 therapy by reducing Foxp3 + CD25 + regulatory T cell (Treg) intratumoural infiltration, and enhancing effector function of CD8 + T cells. L. gallinarum-derived indole-3-carboxylic acid (ICA) was identified as the functional metabolite. Mechanistically, ICA inhibited indoleamine 2,3-dioxygenase (IDO1) expression, therefore suppressing kynurenine (Kyn) production in tumours. ICA also competed with Kyn for binding site on aryl hydrocarbon receptor (AHR) and antagonised Kyn binding on CD4 + T cells, thereby inhibiting Treg differentiation in vitro. ICA phenocopied L. gallinarum effect and significantly improved anti-PD1 efficacy in vivo, which could be reversed by Kyn supplementation.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Cancer immunotherapy using checkpoint blockade
- Record: found
- Abstract: found
- Article: not found
Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors
- Record: found
- Abstract: found
- Article: not found