- Record: found
- Abstract: found
- Article: found
Longitudinal EEG model detects antisense oligonucleotide treatment effect and increased UBE3A in Angelman syndrome
Read this article at
Abstract
Angelman syndrome is a neurodevelopmental disorder caused by deficiency of the maternally inherited UBE3A gene in neurons. Antisense oligonucleotide therapies are under development to reinstate UBE3A protein production. Non-invasive biomarkers to detect target engagement and treatment response are needed to support clinical trials. Delta power measured in the scalp EEG is a reliable biomarker for Angelman syndrome but varies widely across individuals and throughout development, making detection of a treatment effect using single measurements challenging.
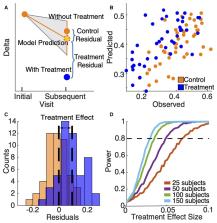
We utilized a longitudinal dataset of 204 EEG recordings from 56 subjects with Angelman syndrome to develop a natural history model of delta (2–4 Hz) power, with predictors of age, elapsed time, and relative delta power at an initial recording. Using this model, we computed the sample and effect sizes needed to detect a treatment effect in a human clinical trial with 80% power. We applied the same model structure to a mouse model of Angelman syndrome ( n = 41) to detect antisense oligonucleotide-mediated treatment effects on absolute delta activity and Ube3a expression. In humans, delta power at a second time point can be reliably predicted using the natural history model. In mice, a treatment effect can be detected after antisense oligonucleotide treatment targeting the Ube3a-antisense transcript through at least 8 weeks post-treatment ( P < 1e-15). Deviations in delta power from the expected natural history correlated with Ube3a expression in the mouse model ( P < 0.001). Deviations in delta power from a human natural history model in Angelman syndrome can detect antisense oligonucleotide-mediated improvement in Ube3a expression in Angelman syndrome mice and may be relevant for human clinical trials.
Abstract
Deviations in delta power from a human natural history model in Angelman syndrome can detect antisense oligonucleotide-mediated improvement in Ube3a expression in Angelman syndrome mice and may be relevant for human clinical trials.
Graphical Abstract
Related collections
Most cited references21
- Record: found
- Abstract: not found
- Article: not found
The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology.
- Record: found
- Abstract: found
- Article: not found
Chronux: a platform for analyzing neural signals.
- Record: found
- Abstract: found
- Article: not found
