- Record: found
- Abstract: found
- Article: found
Activating transcription factor 3 (ATF3) regulates cell growth, apoptosis, invasion and collagen synthesis in keloid fibroblast through transforming growth factor beta (TGF-beta)/SMAD signaling pathway

Read this article at
ABSTRACT
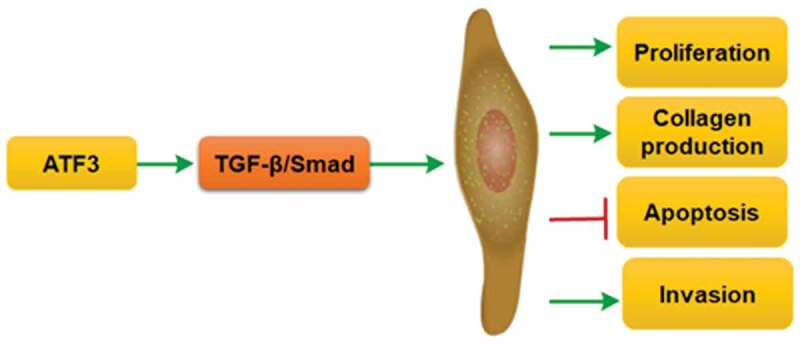
The successful treatment of keloids is a great challenge in the plastic surgery field. Activating transcription factor 3 (ATF3) is discovered as an adaptive responsive gene, which plays a critical role in fibroblast activation. This study aimed to investigate the expression and biological role of ATF3 in the pathogenesis of keloids. ATF3 expression in normal skins and keloids was evaluated by real-time PCR, western blot and immunohistochemistry. Effects of ATF3 on cell growth, apoptosis, invasion and collagen production were evaluated in keloid fibroblast cells overexpressing or downregulating ATF3. ATF3 expression was significantly elevated in keloid tissues when compared with that of normal skins and parakeloidal skin tissues. Moreover, ATF3 promoted cell proliferation and collagen production in keloid fibroblast cells. Conversely, transfection with siRNA targeting ATF3 led to decreased cell viability and collagen synthesis via inhibiting transforming growth factor-β1 (TGF-β1) and fibroblast growth factor 2/8 (FGF2/8) production in keloid fibroblasts. ATF3 could reduce the apoptosis rate of keloid fibroblast cells. Molecularly, we found that ATF3 promoted BCL2 level and inhibit the expression of BCL2 associated agonist of cell death (Bad), Caspase3 and Caspase9 in keloid fibroblast cells. ATF3 also enhanced the invasive potential via upregulating the expression of Matrix Metalloproteinases (MMP) family members (MMP1, MMP2, MMP9 and MMP13). ATF3 could induce activation of TGF-β/Smad signaling pathway in fibroblasts. Collectively, ATF3 could promote growth and invasion, and inhibit apoptosis via TGF-β/Smad pathway in keloid fibroblast cells, suggesting that ATF3 might be considered as a novel therapeutic target for the management of keloid.
GRAPHICAL ABSTRACT
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors
- Record: found
- Abstract: found
- Article: found