- Record: found
- Abstract: found
- Article: found
Why Do We Screen Newborn Infants for Cystic Fibrosis?
editorial
08 July 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The introduction and widespread implementation of newborn bloodspot screening (NBS)
for cystic fibrosis (CF) has offered earlier diagnosis and better outcomes for children
with CF in many countries of the world. It represents a paradigm shift in the diagnostic
pathway for these families. In contrast to a clinical diagnosis, infants are now referred
for diagnostic testing after a positive NBS result and, apart from a small proportion
who present with bowel obstruction (meconium ileus), CF infants have no or only minimal
clinical manifestation of the disease in the early days of life. Clinical symptoms
can appear over the first few weeks, for example, insufficient weight gain, fatty
stools or salt loss syndrome, but are often insidious and difficult to recognise.
The introduction of NBS has enabled the provision of early appropriate treatment (pancreatic
enzyme replacement therapy, fat-soluble vitamins, salt supplementation and antibiotics)
to prevent manifestations of the disease. In the near future, early diagnosis will
facilitate the prompt use of new cystic fibrosis transmembrane conductance regulator
(CFTR) modulator therapies that correct the basic underlying molecular defect.
NBS for CF has been a global success but continues to raise questions with many varied
approaches and the development of new technologies, in particular the ability to undertake
extensive gene examination. It is still valid to ask many questions:
What is the best protocol to achieve high sensitivity and specificity?
Should extensive genetic analysis be part of this algorithm, which enables the identification
of many more CFTR variants?
How to evaluate and manage inconclusive cases with a borderline sweat test or CFTR
variants with unclear clinical relevance?
What is the optimal approach to inform and counsel the parents about the NBS results
and inconclusive findings?
These questions are not easy to answer and require a balanced solution that reflects
the local health care system and may appropriately result in different answers around
the globe.
The aim of this series of articles was to compile the current state of knowledge on
NBS for CF and the questions arising from it. Using the framework of the network of
the Newborn Screening Working Group (NSWG) of the European CF Society (ECFS), we approached
colleagues from all over the world to submit articles for peer review. On the initiative
of the International Journal of Newborn Screening (IJNS), the opportunity arose to
realize this project, and we would like to take this opportunity to thank the authors
for their excellent contributions and the IJNS for their support and cooperation.
We feel the resulting series of articles provides a state-of-the-art evaluation of
the current status of NBS for CF and provides much insight into the questions above
and a path to improve quality across the globe.
The history of newborn bloodspot screening for CF is recorded by Georges Travert and
Mary and Anthony Heeley, all of whom played an important role in these early developments.
They cover the early use of the immune-reactive trypsinogen (IRT) assay, the challenges
they and others faced and how they were overcome [1].
Lutz Naehrlich describes how early diagnosis, multidisciplinary care and optimized
and preventive treatments have improved the outlook for people with CF. From his position
as Director of the European Registry, he is able to give a clear picture of the changing
face of CF, and the direct impact of NBS on this landscape [2].
One of the major challenges in the field of NBS for CF has been the collection of
robust and comparable data across countries and regions. New Zealand was the first
country to establish NBS for CF and Natasha Heather and Dianne Webster are well placed
to reflect on the importance and challenges of collecting the correct metrics [3].
They highlight the critical importance of this if the quality of this public health
initiative is to improve.
Virginie Scotet, Hector Gutierrez and Philip M. Farrell give an overview about the
current situation of NBS for CF across the globe [4]. Each region has typically undertaken
CF NBS after analysis of the advantages, costs and challenges, particularly regarding
the relationship of benefits to risks. The review describes the lessons learned from
the journey toward universal screening wherever CF is prevalent and an analytical
framework for application in those undecided regions.
This complements the next article, in which Rachel Armstrong, Lucy Frith, Fiona Ulph
and Kevin Southern consider NBS for CF from a bioethical perspective [5]. They report
in detail all possible outcomes from NBS for CF and place these in an ethical framework.
Placing these in the context of the genetic profile of the population screened, the
geography of the region and the healthcare resources available, they propose an approach
engaging with stakeholders to determine the best protocol for a region.
Olaf Sommerburg and Jutta Hammermann describe in their review the strengths and weaknesses
of pancreatitis-associated protein (PAP) in the algorithm of NBS for CF [6]. This
biochemical test has emerged as an adjunct to IRT measurement, but the relationship
is complex and is reviewed in detail by these authors who have considerable experience
through implementing this assay as part of the protocol in Germany.
Anne Bergougnoux, Maureen Lopez and Emmanuelle Girodon give a summary of the role
of DNA analysis in the CF screening programme. Their work in the national French laboratory
gives them a unique insight into the challenges of incorporating genetic testing,
especially extended gene analysis (EGA) [7].
A consequence of NBS for CF is the identification of infants with a positive screening
test but an inconclusive diagnostic testing. Anne Munck led the European consensus
exercise to better define the evaluation and management of these infants, in addition
to leading the French research project that monitored outcomes. She places these results
in the context of other work from around the globe [8].
The processing of a positive NBS result for CF not only consists of the screening
part in the laboratory but also the interface between the family and healthcare, and
ultimately the CF team. This is a complex process reviewed by Jürg Barben and Jane
Chudleigh, both of whom have undertaken extensive research projects examining these
issues [9]. It is clear that this is an area that needs considerable improvement across
the globe and the authors review evidence of good practice and propose a roadmap to
improve the quality of this difficult process.
Consistent with the article above is a detailed review of the psychological impact
of NBS for CF by Jane Chudleigh and Holly Chinnery [10]. A better understanding of
the journey that the families of infants with a positive NBS result go on enables
CF teams to predict and ameliorate unnecessary distress.
Again we thank all the authors; there is much to celebrate in the field of NBS for
CF, but clearly still work to do, and this experienced faculty of authors has provided
a series of state-of-the-art articles to help achieve that goal. In addition, we would
like to thank the 19 experts who provided high-quality peer review (sometimes twice)
for this series. We were extremely grateful for their comprehensive and timely contributions,
which were important for the overall quality of the series.
Related collections
Most cited references10

- Record: found
- Abstract: found
- Article: found
Newborn Screening for CF across the Globe— Where Is It Worthwhile ?
- Record: found
- Abstract: found
- Article: found
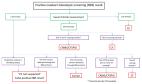
Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis
Anne A Boerema-De Munck (2020)
- Record: found
- Abstract: found
- Article: found
History of Newborn Screening for Cystic Fibrosis—The Early Years
Georges Travert, Mary Heeley, Anthony Heeley (2020)