- Record: found
- Abstract: found
- Article: found
RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF‐κB pathway in oral squamous cell carcinoma
Read this article at
Abstract
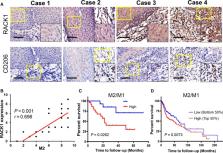
Receptor for activated C kinase 1 (RACK1) has been shown to promote oral squamous cell carcinoma (OSCC) progression, and RACK1 expression levels have been negatively correlated with prognosis in patients with OSCC. Here, we investigated the impact of RACK1 OSCC expression on the recruitment and differentiation of tumor‐associated macrophages. High RACK1 expression in OSCC cells correlated with increased M2 macrophage infiltration in tumor samples from a clinical cohort study. Moreover, the combination of RACK1 expression and the M2/M1 ratio could successfully predict prognosis in OSCC. OSCC cells with high RACK1 expression inhibited the migration of THP‐1 cells, promoted M2‐like macrophage polarization in vitro, and increased the proportion of M2‐like macrophages in a xenograft mouse model. Moreover, both M1‐ and M2‐like macrophage polarization‐associated proteins were induced in macrophages cocultured with RACK1‐silenced cell supernatant. A mechanistic study revealed that the expression and secretion of C‐C motif chemokine 2 (CCL2), C‐C motif chemokine 5 (CCL5), interleukin‐6 (IL‐6), and interleukin‐1 (IL‐1) are closely related to RACK1 expression. In addition, blocking nuclear factor‐kappa B (NF‐κB) could promote M2‐like macrophage polarization. These results indicate that RACK1 and the M2/M1 ratio are predictors of a poor prognosis in OSCC. RACK1 promotes M2‐like polarization by regulating NF‐κB and could be used as a potential therapeutic target for antitumor immunity.
Abstract
High receptor for activated C kinase 1 expression OSCC cells could inhibit the expression and secretion of proinflammatory factors and macrophage chemokines by regulating nuclear factor‐kappa B, thus inhibiting the massive recruitment of macrophages and promoting M2‐like macrophage polarization, inducing a chronic smoldering inflammation microenvironment and promoting the development of tumors.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Cellular metabolism and macrophage functional polarization.
- Record: found
- Abstract: found
- Article: not found
CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis
- Record: found
- Abstract: found
- Article: not found
