- Record: found
- Abstract: found
- Article: found
Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets
Read this article at
Abstract
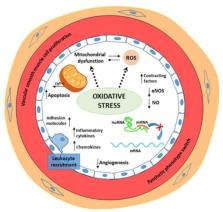
Cardiovascular diseases (CVD), including heart and pathological circulatory conditions, are the world’s leading cause of mortality and morbidity. Endothelial dysfunction involved in CVD pathogenesis is a trigger, or consequence, of oxidative stress and inflammation. Endothelial dysfunction is defined as a diminished production/availability of nitric oxide, with or without an imbalance between endothelium-derived contracting, and relaxing factors associated with a pro-inflammatory and prothrombotic status. Endothelial dysfunction-induced phenotypic changes include up-regulated expression of adhesion molecules and increased chemokine secretion, leukocyte adherence, cell permeability, low-density lipoprotein oxidation, platelet activation, and vascular smooth muscle cell proliferation and migration. Inflammation-induced oxidative stress results in an increased accumulation of reactive oxygen species (ROS), mainly derived from mitochondria. Excessive ROS production causes oxidation of macromolecules inducing cell apoptosis mediated by cytochrome-c release. Oxidation of mitochondrial cardiolipin loosens cytochrome-c binding, thus, favoring its cytosolic release and activation of the apoptotic cascade. Oxidative stress increases vascular permeability, promotes leukocyte adhesion, and induces alterations in endothelial signal transduction and redox-regulated transcription factors. Identification of new endothelial dysfunction-related oxidative stress markers represents a research goal for better prevention and therapy of CVD. New-generation therapeutic approaches based on carriers, gene therapy, cardiolipin stabilizer, and enzyme inhibitors have proved useful in clinical practice to counteract endothelial dysfunction. Experimental studies are in continuous development to discover new personalized treatments. Gene regulatory mechanisms, implicated in endothelial dysfunction, represent potential new targets for developing drugs able to prevent and counteract CVD-related endothelial dysfunction. Nevertheless, many challenges remain to overcome before these technologies and personalized therapeutic strategies can be used in CVD management.
Related collections
Most cited references236
- Record: found
- Abstract: found
- Article: not found
Origins and Mechanisms of miRNAs and siRNAs.
- Record: found
- Abstract: found
- Article: not found
Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease.
- Record: found
- Abstract: found
- Article: not found