- Record: found
- Abstract: found
- Article: found
Drosophila melanogaster as a model to study innate immune memory
Read this article at
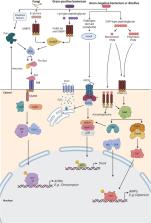
Abstract
Over the last decades, research regarding innate immune responses has gained increasing importance. A growing body of evidence supports the notion that the innate arm of the immune system could show memory traits. Such traits are thought to be conserved throughout evolution and provide a survival advantage. Several models are available to study these mechanisms. Among them, we find the fruit fly, Drosophila melanogaster. This non-mammalian model has been widely used for innate immune research since it naturally lacks an adaptive response. Here, we aim to review the latest advances in the study of the memory mechanisms of the innate immune response using this animal model.
Related collections
Most cited references172
- Record: found
- Abstract: found
- Article: not found
Defining trained immunity and its role in health and disease
- Record: found
- Abstract: found
- Article: not found
Trained immunity: A program of innate immune memory in health and disease.
- Record: found
- Abstract: found
- Article: not found