- Record: found
- Abstract: found
- Article: found
Integrated roles of BclA and DD-carboxypeptidase 1 in Bradyrhizobium differentiation within NCR-producing and NCR-lacking root nodules
Read this article at
Abstract
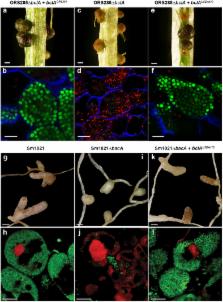
Legumes harbor in their symbiotic nodule organs nitrogen fixing rhizobium bacteria called bacteroids. Some legumes produce Nodule-specific Cysteine-Rich (NCR) peptides in the nodule cells to control the intracellular bacterial population. NCR peptides have antimicrobial activity and drive bacteroids toward terminal differentiation. Other legumes do not produce NCR peptides and their bacteroids are not differentiated. Bradyrhizobia, infecting NCR-producing Aeschynomene plants, require the peptide uptake transporter BclA to cope with the NCR peptides as well as a specific peptidoglycan-modifying DD-carboxypeptidase, DD-CPase1. We show that Bradyrhizobium diazoefficiens strain USDA110 forms undifferentiated bacteroids in NCR-lacking soybean nodules. Unexpectedly, in Aeschynomene afraspera nodules the nitrogen fixing USDA110 bacteroids are hardly differentiated despite the fact that this host produces NCR peptides, suggesting that USDA110 is insensitive to the host peptide effectors and that nitrogen fixation can be uncoupled from differentiation. In agreement with the absence of bacteroid differentiation, USDA110 does not require its bclA gene for nitrogen fixing symbiosis with these two host plants. Furthermore, we show that the BclA and DD-CPase1 act independently in the NCR-induced morphological differentiation of bacteroids. Our results suggest that BclA is required to protect the rhizobia against the NCR stress but not to induce the terminal differentiation pathway.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Plant peptides govern terminal differentiation of bacteria in symbiosis.
- Record: found
- Abstract: found
- Article: not found
Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia.
- Record: found
- Abstract: found
- Article: not found