- Record: found
- Abstract: found
- Article: found
Prospective Evaluation of the Pre-, Intra-, and Postoperative Kinetics of ADAMTS-13, von Willebrand Factor, and Interleukin-6 in Vascular Surgery
Read this article at
Abstract
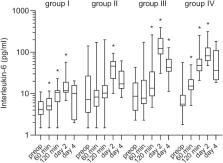
Postoperative thrombotic thrombocytopenic purpura (TTP) shows clinical presentation similar to classical TTP, whereas exact pathophysiological contexts remain unexplained. In this study, we investigated intraoperative and postoperative changes in ADAMTS-13 (a disintegrin and metalloprotease with thrombospondin type 1 motifs, member 13), von Willebrand factor (VWF), large VWF multimers, and interleukin-6 (IL-6) in vascular surgery patients. The objective was to compare the impact of endovascular, peripheral, and aortic surgery on target parameters which are supposed to play a role in surgery-associated TTP. A total of 93 vascular surgery patients were included and divided into 4 groups according to the specific type of intervention they underwent. Blood samples were taken preoperatively, intraoperatively, and postoperatively on days 2 and 4. The ADAMTS-13 activity decreased significantly in 3 of the 4 groups during surgery (from median 81% to 49%, P < .001, in the group undergoing aortoiliacal interventions), whereas the percentage of large VWF multimers increased in all groups of patients. von Willebrand factor antigen increased significantly in all groups on postoperative day 2 and IL-6 increased significantly in the intraoperative and early postoperative period. There was no significant correlation between the intraoperative decrease in ADAMTS-13 and the increase in VWF or IL-6. No patient in this study showed clinical picture of TTP; the precise cause and clinical significance of moderately reduced ADAMTS-13 activity in the perioperative setting have not yet been definitely determined.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura.
- Record: found
- Abstract: not found
- Article: not found
Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura.
- Record: found
- Abstract: found
- Article: not found