- Record: found
- Abstract: found
- Article: found
Effect of a Large Dose of Di (2-ethylhexyl) phthalate (DEHP) on Hepatic Peroxisome in Cynomolgus Monkeys ( Macaca Fascicularis )
Read this article at
Abstract
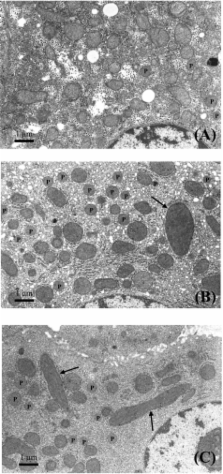
To elucidate the effect of a large dose of di (2-ethylhexyl) phthalate (DEHP), a plasticizer and peroxisome proliferator-activated receptor-α (PPARα) agonist, on hepatic peroxisomes, we orally administered 1,000 mg/kg/day, once daily, to 3 male and 4 female cynomolgus monkeys for 28 days consecutively. Light-microscopic and electron microscopic examinations of the liver were carried out in conjunction with measurement of the hepatic fatty acid β-oxidation system (FAOS), carnitine acetyltransferase (CAT) and carnitine palmitoyltransferase (CPT) activities, which are peroxisomal and/or mitochondrial enzyme activities. Electron microscopically, enlargement of the mitochondria was observed with lamellar orientation of the cristae along the major axis. Although the number of peroxisomes showed a tendency to increase when compared with those in a biopsied specimen before treatment, no abnormality in morphology was observed. A slight increase in CPT activity was noted at termination. No changes were noted in hepatic FAOS or CAT activity. In conclusion, although repeated oral treatment of cynomolgus monkeys with a large dose of DEHP induced a subtle increase in the numbers of peroxisomes with slight enlargements of the mitochondria, this low-sensitivity response to peroxisome proliferators in cynomolgus monkeys was considered to be closer to the response in humans than that in rodents.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand?
- Record: found
- Abstract: found
- Article: not found
A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines.
- Record: found
- Abstract: found
- Article: not found